Abstract
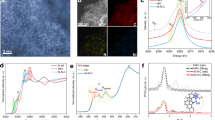
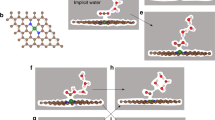
Electrochemical reduction of CO2 to chemical fuel offers a promising strategy for managing the global carbon balance, but presents challenges for chemistry due to the lack of effective electrocatalyst. Here we report atomically dispersed nickel on nitrogenated graphene as an efficient and durable electrocatalyst for CO2 reduction. Based on operando X-ray absorption and photoelectron spectroscopy measurements, the monovalent Ni(i) atomic center with a d9 electronic configuration was identified as the catalytically active site. The single-Ni-atom catalyst exhibits high intrinsic CO2 reduction activity, reaching a specific current of 350 A gcatalyst−1 and turnover frequency of 14,800 h−1 at a mild overpotential of 0.61 V for CO conversion with 97% Faradaic efficiency. The catalyst maintained 98% of its initial activity after 100 h of continuous reaction at CO formation current densities as high as 22 mA cm−2.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Turner, J. A. A realizable renewable energy future. Science 285, 687–689 (1999).
Graves, C., Ebbesen, S. D., Mogensen, M. & Lackner, K. S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 15, 1–23 (2011).
Gao, S. et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 529, 68–71 (2016).
Zhang, Y.-J., Sethuraman, V., Michalsky, R. & Peterson, A. A. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts. ACS Catal. 4, 3742–3748 (2014).
Kim, D., Resasco, J., Yu, Y., Asiri, A. M. & Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 5, 4948 (2014).
Martin, A. J., Larrazabal, G. O. & Perez-Ramirez, J. Towards sustainable fuels and chemicals through the electrochemical reduction of CO2: lessons from water electrolysis. Green. Chem. 17, 5114–5130 (2015).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Zhu, W. et al. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 135, 16833–16836 (2013).
Li, C. W. & Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012).
Liu, J. Catalysis by supported single metal atoms. ACS Catal. 7, 34–59 (2017).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Yang, M. et al. Catalytically active Au-O(OH)x species stabilized by alkali ions on zeolites and mesoporous oxides. Science 346, 1498–1501 (2014).
Back, S. et al. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 8, 1090–1096 (2017).
Zhao, C. et al. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 139, 8078–8081 (2017).
Jia, Q. et al. Experimental observation of redox-induced Fe–N switching behavior as a determinant role for oxygen reduction activity. ACS Nano 9, 12496–12505 (2015).
Yang, H. B. et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2, e1501122 (2016).
Kabir, S., Artyushkova, K., Serov, A., Kiefer, B. & Atanassov, P. Binding energy shifts for nitrogen-containing graphene-based electrocatalysts—experiments and DFT calculations. Surf. Interface Anal. 48, 293–300 (2016).
Lin, Y.-C. et al. Structural and chemical dynamics of pyridinic-nitrogen defects in graphene. Nano Lett. 15, 7408–7413 (2015).
Colpas, G. J. et al. X-ray spectroscopic studies of nickel complexes, with application to the structure of nickel sites in hydrogenases. Inorg. Chem. 30, 920–928 (1991).
Wongnate, T. et al. The radical mechanism of biological methane synthesis by methyl-coenzyme M reductase. Science 352, 953–958 (2016).
Xu, S. et al. Dynamic structural changes of SiO2 supported Pt–Ni bimetallic catalysts over redox treatments revealed by NMR and EPR. J. Phys. Chem. C. 119, 21219–21226 (2015).
Brazzolotto, D. et al. Nickel-centred proton reduction catalysis in a model of [NiFe] hydrogenase. Nat. Chem. 8, 1054–1060 (2016).
Ebner, S., Jaun, B., Goenrich, M., Thauer, R. K. & Harmer, J. Binding of coenzyme B induces a major conformational change in the active site of methyl-coenzyme M reductase. J. Am. Chem. Soc. 132, 567–575 (2010).
Matsubara, K. et al. Monomeric three-coordinate N-heterocyclic carbene nickel(I) complexes: synthesis, structures, and catalytic applications in cross-coupling reactions. Organometallics 35, 3281–3287 (2016).
Suh, M. P., Kim, H. K., Kim, M. J. & Oh, K. Y. Synthesis, properties, and X-ray structures of nickel(I) complexes of polyaza macrotricyclic ligands. Inorg. Chem. 31, 3620–3625 (1992).
Avakyan, L. A. et al. Atomic structure of nickel phthalocyanine probed by X-ray absorption spectroscopy and density functional simulations. Opt. Spectrosc. 114, 347–352 (2013).
Yamamoto, T. Assignment of pre-edge peaks in K-edge x-ray absorption spectra of 3d transition metal compounds: electric dipole or quadrupole? X-Ray Spectrom. 37, 572–584 (2008).
Douglas, C. D., Dias, A. V. & Zamble, D. B. The metal selectivity of a short peptide maquette imitating the high-affinity metal-binding site of E. coli HypB. Dalton Trans. 41, 7876–7878 (2012).
Rossi, G., d'Acapito, F., Amidani, L., Boscherini, F. & Pedio, M. Local environment of metal ions in phthalocyanines: K-edge X-ray absorption spectra. Phys. Chem. Chem. Phys. 18, 23686–23694 (2016).
Sarangi, R., Dey, M. & Ragsdale, S. W. Geometric and electronic structures of the NiI and methyl−NiIII intermediates of methyl-coenzyme M reductase. Biochemistry 48, 3146–3156 (2009).
Marken, F., Neudeck, A. & Bond, A. M. in Electroanalytical Methods: Guide to Experiments and Applications (ed. Scholz, F.) 57–106 (Springer, Berlin, 2010).
Gattrell, M., Gupta, N. & Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 594, 1–19 (2006).
Kim, C. et al. Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J. Am. Chem. Soc. 137, 13844–13850 (2015).
Menges, F. S. et al. Capture of CO2 by a cationic nickel(I) complex in the gas phase and characterization of the bound, activated CO2 molecule by cryogenic ion vibrational predissociation spectroscopy. Angew. Chem. Int. Ed. 55, 1282–1285 (2016).
Fujita, E., Furenlid, L. R. & Renner, M. W. Direct XANES evidence for charge transfer in Co−CO2 complexes. J. Am. Chem. Soc. 119, 4549–4550 (1997).
Sakaki, S. Can carbon dioxide coordinate to a nickel(I) complex? An ab initio MO/SD-CI study. J. Am. Chem. Soc. 112, 7813–7814 (1990).
Ding, X. et al. Interaction of carbon dioxide with Ni(110): A combined experimental and theoretical study. Phys. Rev. B 76, 195425 (2007).
Freund, H. J. & Roberts, M. W. Surface chemistry of carbon dioxide. Surf. Sci. Rep. 25, 225–273 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dean, J. A. Lange’s Handbook of Chemistry Ch. 9 (McGraw-Hill, New York, 1979).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Acknowledgements
We would like to acknowledge funding support from the National Key Projects for Fundamental Research and Development of China (2016YFA0202804), the Strategic Priority Research Programme of the Chinese Academy of Sciences (XDB17020400), Singapore Ministry of Education Academic Research Fund (AcRF) Tier 1: RG10/16 and RG111/15, Singapore A*Star Science and Engineering Research Council—Public Sector Funding (PSF): 1421200075, the National Research Foundation (NRF), Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.
Author information
Authors and Affiliations
Contributions
H.B.Y., Y.H., T.Z. and B.L. conceived and designed the project. S.H., H.W. and H.M.C performed the X-ray absorption experiments. H.B.Y., S.L., J.G. and C.L. performed the electrochemical experiments and analyzed the electrochemical data. K.Y. and W.C. conducted the photoelectron spectroscopy measurements. S.M., L.Z. and R.C contributed to the structure characterizations. X.H., W.C. and X.Y. carried out the theoretical calculations. H.B.Y., Y.H., T.Z. and B.L. analyzed the experimental data and prepared the manuscript. All authors reviewed and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Supplementary Tables 1–5 and Supplementary References
Rights and permissions
About this article
Cite this article
Yang, H., Hung, SF., Liu, S. et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat Energy 3, 140–147 (2018). https://doi.org/10.1038/s41560-017-0078-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-017-0078-8
This article is cited by
-
Design and diagnosis of high-performance CO2-to-CO electrolyzer cells
Nature Chemical Engineering (2024)
-
Photochemical tuning of dynamic defects for high-performance atomically dispersed catalysts
Nature Materials (2024)
-
Constructing sulfur and oxygen super-coordinated main-group electrocatalysts for selective and cumulative H2O2 production
Nature Communications (2024)
-
Water-participated mild oxidation of ethane to acetaldehyde
Nature Communications (2024)
-
Nanocurvature-induced field effects enable control over the activity of single-atom electrocatalysts
Nature Communications (2024)