Abstract
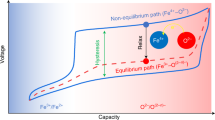
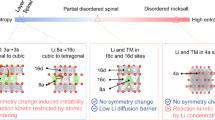
The Li-rich rocksalt oxides Li2MO3 (M = 3d/4d/5d transition metal) are promising positive-electrode materials for Li-ion batteries, displaying capacities exceeding 300 mAh g–1 thanks to the participation of the oxygen non-bonding O(2p) orbitals in the redox process. Understanding the oxygen redox limitations and the role of the O/M ratio is therefore crucial for the rational design of materials with improved electrochemical performances. Here we push oxygen redox to its limits with the discovery of a Li3IrO4 compound (O/M = 4) that can reversibly take up and release 3.5 electrons per Ir and possesses the highest capacity ever reported for any positive insertion electrode. By quantitatively monitoring the oxidation process, we demonstrate the material’s instability against O2 release on removal of all Li. Our results show that the O/M parameter delineates the boundary between the material’s maximum capacity and its stability, hence providing valuable insights for further development of high-capacity materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Koga, H. et al. Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J. Electrochem. Soc. 160, A786–A792 (2013).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015).
Freire, M. et al. A new active Li–Mn–O compound for high energy density Li-ion batteries. Nat. Mater. 15, 173–177 (2016).
Saubanère, M., McCalla, E., Tarascon, J.-M. & Doublet, M.-L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 9, 984–991 (2016).
Xie, Y., Saubanère, M. & Doublet, M.-L. Requirements for reversible extra-capacity in Li-rich layered oxides for Li-ion batteries. Energy Environ. Sci. 10, 266–274 (2017).
Seo, D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
McCalla, E. et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Pearce, P. E. et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode β-Li2IrO3. Nat. Mater. 16, 580–586 (2017).
Yabuuchi, N., Tahara, Y., Komaba, S., Kitada, S. & Kajiya, Y. Synthesis and electrochemical properties of Li4MoO5–NiO binary system as positive electrode materials for rechargeable lithium batteries. Chem. Mater. 28, 416–419 (2016).
Cabana, J., Monconduit, L., Larcher, D. & Palacín, M. R. beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 22, E170–E192 (2010).
Dahn, J. R., von Sacken, U. & Michal, C. A. Structure and electrochemistry of Li1±yNiO2 and a new Li2NiO2 phase with the Ni(OH)2 structure. Solid State Ion. 44, 87–97 (1990).
Moreau, P., Guyomard, D., Gaubicher, J. & Boucher, F. Structure and stability of sodium intercalated phases in olivine FePO4. Chem. Mater. 22, 4126–4128 (2010).
Qi, B., Perez, I., Ansari, P. H., Lu, F. & Croft, M. L2 and L3 measurements of transition-metal 5d orbital occupancy, spin-orbit effects, and chemical bonding. Phys. Rev. B 36, 2972–2975 (1987).
Choy, J.-H., Kim, D.-K., Demazeau, G. & Jung, D.-Y. LIII-edge XANES study on unusually high valent iridium in a perovskite lattice. J. Phys. Chem. 98, 6258–6262 (1994).
Choy, J.-H., Kim, D.-K., Hwang, S.-H., Demazeau, G. & Jung, D.-Y. XANES and EXAFS studies on the Ir-O bond covalency in ionic iridium perovskites. J. Am. Chem. Soc. 117, 8557–8566 (1995).
Zhu, Z. et al. Anion-redox nanolithia cathodes for Li-ion batteries. Nat. Energy 1, 16111 (2016).
Jacquet, Q. et al. The Li3RuyNb1–yO4 (0≤y≤1) system: structural diversity and Li insertion and extraction capabilities. Chem. Mater. 29, 5331–5343 (2017).
Kim, S. et al. Material design of high-capacity Li-rich layered-oxide electrodes: Li2MnO3 and beyond. Energy Environ. Sci. 10, 2201–2211 (2017).
Leriche, J. B. et al. An electrochemical cell for operando study of lithium batteries using synchrotron radiation. J. Electrochem. Soc. 157, A606–A610 (2010).
Lepoivre, F., Grimaud, A., Larcher, D. & Tarascon, J.-M. Long-time and reliable gas monitoring in Li-O2 batteries via a Swagelok derived electrochemical cell. J. Electrochem. Soc. 163, A923–A929 (2016).
Briois, V. et al. ROCK: the new Quick-EXAFS beamline at SOLEIL. J. Phys. Conf. Ser. 712, 012149 (2016).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Massart, B. et al. Handbook of Chemometrics and Qualimetrics (Elsevier, 1997).
Juan, A., de, Jaumot, J. & Tauler, R. Multivariate curve resolution (MCR). Solving the mixture analysis problem. Anal. Methods 6, 4964–4976 (2014).
Alexander, A. et al. Structural and magnetic properties of Li3RuO4. J. Mater. Chem. 13, 2612–2616 (2003).
Clancy, J. P. et al. Spin-orbit coupling in iridium-based 5d compounds probed by x-ray absorption spectroscopy. Phys. Rev. B 86, 195131 (2012).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Anisimov, I., Zaanen, J. & Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943–954 (1991).
Acknowledgements
We thank P. Pearce for providing the β-Li2IrO3 and L. Lemarquis for helping in the DEMS experiment. We are particularly grateful to S. Belin, V. Briois and L. Stievano for helpful discussions on XAS analysis and synchrotron SOLEIL (France) for providing beamtime at the ROCK beamline (financed by the French National Research Agency (ANR) as part of the ‘Investissements d’Avenir’ programme, reference: ANR-10-EQPX-45). A.J.P and A.I. acknowledge the GdR C(RS)2 for the workshop organized on a chemometric approach for XAS data analysis. V. Nassif is acknowledged for her help during neutron diffraction experiments performed at Institut Laue Langevin on D1B. Use of the 11-BM mail service of the APS at Argonne National Laboratory was supported by the US Department of Energy under contract No. DE-AC02-06CH11357 and is gratefully acknowledged. This work has been performed with the support of the European Research Council (ERC) (FP/2014)/ERC Grant-Project 670116-ARPEMA.
Author information
Authors and Affiliations
Contributions
A.J.P. carried out the synthesis; A.J.P., Q.J., D.L. and J.-M.T. designed and performed the electrochemical studies; A.J.P., Q.J. and G.R. performed the diffraction experiments and analysis; A.J.P., Q.J. and A.I. carried out the X-ray absorption study; D.B. collected and analysed the TEM images; H.V. collected and analysed the EPR spectra; M.S. and M.-L.D. conducted the DFT study; A.J.P. and J.-M.T. wrote the manuscript and all authors discussed the experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Supplementary Figures 1–13
Rights and permissions
About this article
Cite this article
Perez, A.J., Jacquet, Q., Batuk, D. et al. Approaching the limits of cationic and anionic electrochemical activity with the Li-rich layered rocksalt Li3IrO4 . Nat Energy 2, 954–962 (2017). https://doi.org/10.1038/s41560-017-0042-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-017-0042-7
This article is cited by
-
Stabilizing lattice oxygen redox in layered sodium transition metal oxide through spin singlet state
Nature Communications (2023)
-
Origin and regulation of oxygen redox instability in high-voltage battery cathodes
Nature Energy (2022)
-
Correlating ligand-to-metal charge transfer with voltage hysteresis in a Li-rich rock-salt compound exhibiting anionic redox
Nature Chemistry (2021)
-
Nonpolarizing oxygen-redox capacity without O-O dimerization in Na2Mn3O7
Nature Communications (2021)
-
The role of O2 in O-redox cathodes for Li-ion batteries
Nature Energy (2021)