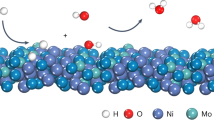
Abstract
The production of pure hydrogen for use in energy applications and related industries often relies on the permeation of hydrogen through palladium-based membranes. However, the scarcity of Pd reserves necessitates the development of affordable alternatives with high hydrogen permeability. Here we report room-temperature hydrogen permeability of titanium nitrides (widely used as tough and inert coating materials) enabled by mixed hydride ion–electron conductivity. Combined spectroscopic, permeability and microgravimetric measurements reveal that nanocrystalline TiN x membranes feature enhanced grain-boundary diffusion of hydride anions associated with interfacial Ti cations on nanograins. Since the corresponding activation energies are very low (<10 kJ mol–1), these membranes yield a considerably higher room-temperature hydrogen flux than Pd membranes of equivalent thickness. Overall, the current study establishes general guidelines for developing hydride ion transport membranes based on a simple transition metal nitride for hydrogen purification, membrane reactors and other applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Adams, B. D. & Chen, A. The role of palladium in a hydrogen economy. Mater. Today 14, 282–289 (2011).
Cornaglia, L., Munera, J. & Lombardo, E. Recent advances in catalysts, palladium alloys and high temperature WGS membrane reactors: A review. Int. J. Hydrogen Energy 40, 3423–3437 (2015).
Al-Mufachi, N. A., Rees, N. V. & Wilkens, R. S. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sust. Energ. Rev. 47, 540–551 (2015).
Rebollo, E. et al. Exceptional hydrogen permeation of all-ceramic composite robust membranes based on BaCe0.65Zr0.20Y0.15O3-δ and Y- or Gd-doped ceria. Energy Environ. Sci. 8, 3675–3686 (2015).
Escolastico, S. et al. Enhanced H2 separation through mixed proton-electron conducting membranes based on La5.5W0.8M0.2O11.25-δ. Chem. Sus. Chem 6, 1523–1532 (2013).
Yao, J. & Wang, H. Zeolitic imidazolate framework composite membranes and thin films: synthesis and applications. Chem. Soc. Rev. 43, 4470–4493 (2014).
Song, S. J., Wachsman, E. D., Rhodes, J., Dorris, S. E. & Balachandran, U. Hydrogen permeability of SrCe1−x M x O3−δ (x=0.05, M=Eu, Sm). Solid State Ionics 167, 99–105 (2004).
Zhu, Z. et al. Evaluation of hydrogen permeation properties of Ni-Ba(Zr0.7Pr0.1Y0.2)O3-δ cermet membranes. Int. J. Hydrogen Energy 39, 11683–11689 (2014).
Bouwmeester, H. J. M. & Burggraaf, A. J. in Handbook of Solid State Electrochemistry (eds Gellings, P. J. & Bouwmeester, H. J. M.) (CRC Press, Boca Raton, 1997).
Kreuer, K. D. Proton-conducting oxides. Annu. Rev. Mater. Res. 33, 333–359 (2003).
Yamazaki, Y. et al. Proton trapping in yttrium-doped barium zirconate. Nat. Mater. 12, 647–651 (2013).
Kim, D., Miyoshi, S., Tsuchiya, T. & Yamaguchi, S. Percolation conductivity of BaZrO3-BaFeO3 solid solutions. Solid State Ionics 262, 875–878 (2014).
Zhou, Y. et al. Strongly correlated perovskite fuel cells. Nature 534, 231–234 (2016).
de Vos, R. M. & Verweij, H. High-selectivity, high-flux silica membranes for gas separation. Science 279, 1710–1711 (1998).
Uhlhorn, R. J. R., Zaspalis, V. T., Keizer, K. & Burggraaf, A. J. Synthesis of ceramic membranes. J. Mater. Sci. 27, 527–552 (1992).
Ramana, C. V., White, S., Esparza, N., Rangel, V. & Campbell, A. L. Crystal structure and morphology of nanocrystalline TiN thin films. J. Electron. Mater. 41, 3139–3144 (2012).
Holleck, G. L. Diffusion and solubility of hydrogen in palladium and palladium-silver alloys. J. Phys. Chem. 74, 503–511 (1970).
Bonanos, N., Huijser, A. & Poulsen, F. W. H/D isotope effects in high temperature proton conductors. Solid State Ionics 275, 9–13 (2015).
Kim, S. et al. On the conduction pathway for protons in nanocrystalline yttria-stabilized zirconia. Phys. Chem. Chem. Phys. 11, 3035–3038 (2009).
Schlom, D. G., Chen, L.-Q., Pan, X., Schmehl, A. & Zurbuchen, M. A. A thin film approach to engineering functionality into oxide. J. Am. Ceram. Soc. 91, 2429–2454 (2008).
Aoki, Y., Hashizume, M., Onoue, S. & Kunitake, T. Determination of surface area and porosity of small, nanometer-thick films by quartz crystal microbalance measurement of gas adsorption. J. Phys. Chem. B 112, 14578–14582 (2008).
Uekawa, N. & Kaneko, K. Nonstoichiometric properties of nanoporous iron oxide films. J. Phys. Chem. B 102, 8719–8724 (1998).
Pierson, H. O. Handbook of Chemical Vapor Deposition: Principle, Technology and Applications (2nd edn) (Noyes Publications, New York, 1999).
Hara, S. et al. Hydrogen diffusion coefficient and mobility in palladium as a function of equilibrium pressure evaluated by permeation measurement. J. Memb. Sci. 421, 355–360 (2015).
Gelhausen, O. et al. Dissociation of H-related defect complexes in Mg-doped GaN. Phys. Rev. B 69, 125210 (2004).
Kleekajai, S. et al. Vibrational properties of the H-N-H complex in dilute III-N-V alloys: Infrared spectroscopy and density functional theory. Phys. Rev. B 29, 085213 (2008).
Suihkonen, S., Pimputkar, S., Speck, J. S. & Nakamura, S. Infrared absorption of hydrogen-related defects in ammonothermal GaN. Appl. Phys. Lett. 108, 202105 (2016).
Janotti, A., Zhang, S. B. & Wei, S.-H. Hydrogen vibration modes in GaP:N: the pivotal role of nitrogen in stabilizing the H* 2 complex. Phys. Rev. Lett. 88, 125506 (2002).
Janotti, A., Zhang, S. B. & Wei, S.-H. Effects of hydrogen on the electronic properties of dilute GaAsN alloys. Phys. Rev. Lett. 88, 086403 (2002).
Van de Walle, C. G. & Neugebauer, J. Universal alignment of hydrogen levels in semiconductors, insulators and solutions. Nature 423, 626–628 (2003).
Holbech, J. D., Nielsen, B. B., Jones, R., Sitch, P. & Öberg, S. H2* defect in crystalline silicon. Phys. Rev. Lett. 71, 875–878 (1993).
Sun, W. et al. Heterogeneous reduction of carbon dioxide by hydride-terminated silicon nanocrystals. Nat. Commun. 7, 125531 (2016).
Fujii, R., Gotoh, Y., Liao, M. Y., Tsuji, H. & Ishikawa, J. Work function measurement of transition metal nitride and carbide thin films. Vacuum 80, 832–835 (2006).
Hood, D. M., Pitzer, R. & Schaefer, H. Electronic structure of homoleptic transition metal hydrides: TiH4, VH4, CrH4, MnH4, FeH4, CoH4, and NiH4. J. Chem. Phys. 71, 705–712 (1979).
Tsuchiya, B. et al. Electronic structure of the bulk of titanium hydrides fractured in ultrahigh vacuum by XPS surface analysis. J. Surf. Anal. 14, 424–427 (2008).
Yan, Y. et al. The formation of Ti-H species at interface is lethal to the efficiency of TiO2-based dye-synthesized devices. J. Am. Chem. Soc. 139, 2083–2089 (2017).
Roesky, H. W., Bai, Y. & Noltemeyer, M. Synthesis and structure of [{(η5-C5Me5)Ti(NH)}3N], a titanium imide nitride. Angew. Chem. Int. Ed. 28, 754–755 (1989).
Abarca, A. et al. Ammonolysis of mono(pentamethylcyclopentadienyl) titanium(IV) derivatives. Inorg. Chem. 39, 642–651 (2000).
Hayashi, K., Sushko, P. V., Hashimoto, Y., Shluger, A. L. & Hosono, H. Hydrogen ion in oxide hosts hidden by hydroxide ions. Nat. Commun. 5, 35151 (2014).
Froben, F. W. & Rogge, F. Matrix infrared measurement of TiN. Chem. Phys. Lett. 78, 264–265 (1981).
Nyquis, R. A. & Kagel, R. O. Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts. (Academic Press, London, 1971).
Dementjef, A. P. et al. X-ray photoelectron spectroscopy refernce data for identification of C3N4 phase in carbon-nitogen films. Diamond Related Mater. 9, 1904–1907 (2000).
Chertihin, G. V. & Andrews, L. Reactions of laser ablated Ti atoms with hydrogen during condensation in excess argon. Infrared spectra of the TiH, TiH2, TiH3 and TiH4 molecules. J. Am. Chem. Soc. 116, 8322–8327 (1994).
Xiao, Z. L., Hauge, R. H. & Margrave, J. L. Reaction of vanadium and titanium with molecular hydrogen in Kr and Ar matrices at 12 K. J. Phys. Chem. 95, 2696–2700 (1991).
Siodmiak, M. et al. Theoretical study of hydrogen adsorption and diffusion on TiN (100) surface. Phys. Stat. Sol. B 226, 29–36 (2001).
Mario, M. & Milman, V. Density-functional study of bulk and surface properties of titanium nitride using different exchange-correlation functionals. Phys. Rev. B 62, 2899 (2000).
Conway, B. E. Two dimensional and quasi-two-dimensional isotherms for Li intercalation and upd processes at surfaces. Electrochim. Acta 38, 1249–1258 (1993).
Kobayashi, G. et al. Pure H− conduction in oxyhydrides. Science. 351, 1314–1317 (2016).
Verbraeken, M. C., Cheung, C., Suard, E. & Irvine, J. T. S. High H− ionic conductivity in barium hydride. Nat. Mater. 14, 95–100 (2014).
Maier, J. Nanoionics: ion transport and electrochemical storage in confined systems. Nat. Mater. 4, 805–815 (2005).
Ramanathan, S. Interface-mediated ultrafast carrier conduction in oxide thin films and superlattices for energy. J. Vac. Sci. Technol. A 27, 1126–1134 (2009).
Krukau, A. V., Vydrov, O. A., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006).
Siodmiak, M. et al. Theoretical study of hydrogen adsorption and diffusion on TiN(100) surface. Phys. Stat. Sol. 226, 29–36 (2001).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mat. Sci 6, 15 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Persson, C., Zhao, Y.-J., Lany, S. & Zunger, A. n-type doping of CuInSe2 and CuGaSe2. Phys. Rev. B 72, 035211 (2005).
Iwazaki, Y., Suzuki, T. & Tsuneyuki, S. Negatively charged hydrogen at oxygen-vacancy sites in BaTiO3: Density functional calculation. J. Appl. Phys. 108, 083705 (2010).
Climent-Font, A., Wätjen, U. & Bax, H. Quantitative RBS analysis using RUMP. On the accuracy of the He stopping in Si. Nucl. Instrum. Methods B 71, 81–86 (1992).
Itoh, N., Wu, T. H. & Haraya, K. Two- and three-dimensional analysis of diffusion through a dense membrane supported on a porous material. J. Membr. Sci. 99, 175–183 (1999).
Acknowledgements
This work was supported by the PRESTO `Creation of Innovative Core Technology for Manufacture and Use of Energy Carriers from Renewable Energy’ project, funded by Japan Science and Technology Agency, Japan and the `Nanotechnology Platform’ programme of the MEXT Japan. C.K. was supported by the MEXT Japan through the programme for Leading Graduate Schools (Hokkaido University `Ambitious Leader’s Program’).
Author information
Authors and Affiliations
Contributions
Y.A. designed the present work; Y.A. and C.K. prepared the manuscript; C.K. performed the membrane fabrications, electron microscopy, FT-IR, QCM and NMR measurements and hydrogen permeation tests with supervision from Y.A., and discussed the data with Y.A., E.T., C.Z. and H.H.; SIMS measurements were performed by C.K., R.S. and M.M.; C.K. and Y.K. conducted the density functional theory calculations; Y.A. and S.N. performed Rutherford back scattering measurements.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Figures 1–10, Supplementary Tables 1–4 and Supplementary References
Rights and permissions
About this article
Cite this article
Kura, C., Kunisada, Y., Tsuji, E. et al. Hydrogen separation by nanocrystalline titanium nitride membranes with high hydride ion conductivity. Nat Energy 2, 786–794 (2017). https://doi.org/10.1038/s41560-017-0002-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-017-0002-2
This article is cited by
-
General molten-salt route to three-dimensional porous transition metal nitrides as sensitive and stable Raman substrates
Nature Communications (2021)
-
Review: recent progress in low-temperature proton-conducting ceramics
Journal of Materials Science (2019)