Abstract
Burgess Shale-type faunas are critical to our understanding of animal evolution during the Cambrian, giving an unrivalled view of the morphology of ancient organisms and the ecology of the earliest animal-dominated communities. Rare examples in Lower Ordovician strata such as the Fezouata Biota illustrate the subsequent evolution of ecosystems but only from before the main phase of the Great Ordovician Biodiversification Event. Later Ordovician Konservat-Lagerstätten are not directly comparable with the Burgess Shale-type faunas as they do not represent diverse, open-shelf communities, limiting our ability to track ecological development through the critical Ordovician biodiversification interval. Here we present the Castle Bank fauna: a highly diverse Middle Ordovician Burgess Shale-type fauna from Wales (UK) that is directly comparable with the Burgess Shale and Chengjiang biotas in palaeoenvironment and preservational style. The deposit includes animals with morphologies similar to the iconic Cambrian taxa Opabinia, Yohoia and Wiwaxia, combined with early examples of more derived groups such as barnacles. Many taxa such as kinorhynchs show the small sizes typical of modern faunas, illustrating post-Cambrian miniaturization. Castle Bank provides a new perspective on early animal evolution, revealing the next chapter in ecosystem development following the Chengjiang, Burgess Shale and Fezouata biotas.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Numerical data sharing is not applicable to this paper as no datasets were generated or analysed during the current study. Figured material has been deposited in Amgueddfa Cymru—Museum Wales, Cardiff, UK (prefix NMW) and the Nanjing Institute of Geology and Palaeontology, Nanjing, China (prefix NIGP), with SCF microfossils deposited in the Museum of Evolution, Uppsala, Sweden (prefix PMU). To protect the site and at the request of the landowner, locality details are not being published. All necessary information has been deposited with the specimens in Amgueddfa Cymru—Museum Wales, where it is available to bona fide researchers.
References
Zhang, X., Liu, W. & Zhao, Y. Cambrian Burgess shale-type Lagerstätten in south China: distribution and significance. Gondwana Res. 14, 255–262 (2008).
Holmes, J. D., García-Bellido, D. C. & Lee, M. S. Y. Comparisons between Cambrian Lagerstätten assemblages using multivariate, parsimony and Bayesian methods. Gondwana Res. 55, 30–41 (2018).
Muscente, D. et al. Exceptionally preserved fossil assemblages through geologic time and space. Gondwana Res. 48, 164–188 (2017).
Gaines, R. R. et al. Mechanism for Burgess Shale-type preservation. Proc. Natl Acad. Sci. USA 109, 5180–5184 (2012).
Gaines, R. R. Burgess Shale-type preservation and its distribution in space and time. Paleontol. Soc. Pap. 20, 123–146 (2014).
Van Roy, P. et al. Ordovician faunas of Burgess Shale type. Nature 465, 215–218 (2010).
Botting, J. P., Muir, L. A., Jordan, N. & Upton, C. An Ordovician variation on Burgess Shale-type biotas. Sci. Rep. 5, 9947 (2015).
Van Roy, P., Briggs, D. E. G. & Gaines, R. R. The Fezouata fossils of Morocco; an extraordinary record of marine life in the Early Ordovician. J. Geol. Soc. 172, 541–549 (2015).
Saleh, F. et al. Taphonomic bias in exceptionally preserved biotas. Earth Planet. Sci. Lett. 529, 115873 (2020).
Botting, J. P. Hexactins in the ‘protomonaxonid’ sponge Choiaella and proposal of Ascospongiae (class nov.) as a formal replacement for the Protomonaxonida. Bull. Geosci. 96, 265–277 (2021).
Van Roy, P., Daley, A. C. & Briggs, D. E. G. Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522, 77–80 (2015).
Vinther, J., Parry, L., Briggs, D. E. G. & Van Roy, P. Ancestral morphology of crown-group molluscs revealed by a new Ordovician stem aculiferan. Nature 542, 471–474 (2017).
Pates, S., Botting, J. P., McCobb, L. M. E. & Muir, L. A. A miniature Ordovician hurdiid from Wales demonstrates the adaptability of Radiodonta. R. Soc. Open Sci. 7, 200459 (2020).
Farrell, Ú. C., Martin, M. J., Hagadorn, J. W., Whiteley, T. & Briggs, D. E. G. Beyond Beecher’s Trilobite bed: widespread pyritization of soft tissues in the Late Ordovician Taconic foreland basin. Geology 37, 907–910 (2009).
Young, G. A. et al. Great Canadian Lagerstätten 3. Late Ordovician Konservat-Lagerstätten in Manitoba. Geosci. Can. 39, 201–213 (2012).
Gabbott, S. E., Browning, C., Theron, J. N. & Whittle, R. J. The late Ordovician Soom Shale Lagerstätte: an extraordinary post-glacial fossil and sedimentary record. J. Geol. Soc. https://doi.org/10.1144/jgs2016-076 (2017).
Briggs, D. E. G., Liu, H. P., McKay, R. M. & Witzke, B. J. The Winneshiek biota: exceptionally well-preserved fossils in a Middle Ordovician impact crater. J. Geol. Soc. 175, 865–874 (2018).
Hearing, T. W. et al. Survival of Burgess Shale-type animals in a Middle Ordovician deep-water setting. J. Geol. Soc. 173, 628–633 (2016).
Bevins, R. E., Lees, G. J. & Roacht, R. A. Petrogenesis of Ordovician igneous rocks in the southern part of the Welsh Basin. Geol. Mag. 129, 615–624 (1992).
Cocks, L. R. M. & Torsvik, T. H. Ordovician palaeogeography and climate change. Gondwana Res. 100, 53–72 (2021).
Davies, J. R., Fletcher, C. J. N., Waters, R. A. & Wilson, D. Geology of the Country Around Llanilar and Rhayader: Memoir for 1:50 000 Geological Sheets 178 and 179 (England and Wales) (HM Stationery Office, 1997).
Gradstein, F., Ogg, J., Schmitz, M. & Ogg, G. (eds) The Geologic Time Scale (Elsevier, 2012).
Pates, S., Botting, J. P., Muir, L. A. & Wolfe, J. M. Ordovician opabiniid-like animals and the role of the proboscis in euarthropod head evolution. Nat. Commun. 13, 6969 (2022).
Botting, J. P. & Ma, J. Y. A probable hyalonematid sponge (Hexactinellida: Amphidiscophora) from the Middle Ordovician of the Builth Inlier, Wales. Palaeoworld 31, 621–632 (2022).
Yang, X. et al. A juvenile-rich palaeocommunity of the lower Cambrian Chengjiang biota sheds light on palaeo-boom or palaeo-bust environments. Nat. Ecol. Evol. 5, 1082–1090 (2021).
Tanaka, G., Hou, X., Ma, X., Edgecombe, G. D. & Strausfeld, N. J. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502, 364–367 (2013).
Olesen, J., Haug, J. T., Maas, A. & Waloszek, D. External morphology of Lightiella monniotae (Crustacea, Cephalocarida) in the light of Cambrian ‘Orsten’ crustaceans. Arthropod Struct. Dev. 40, 449–478 (2011).
Butterfield, N. J. & Harvey, T. H. P. Small carbonaceous fossils (SCFs): a new measure of early Paleozoic paleobiology. Geology 40, 71–74 (2012).
Wallet, E., Slater, B. J., Willman, S. & Peel, J. S. Small carbonaceous fossils (SCFs) from North Greenland: new light on metazoan diversity in early Cambrian shelf environments. Pap. Palaeontol. 7, 1403–1433 (2021).
Wolfe, J. M., Daley, A. C., Legg, D. A. & Edgecombe, G. D. Fossil calibrations for the arthropod Tree of Life. Earth Sci. Rev. 160, 43–110 (2016).
Caron, J. B. & Jackson, D. A. Paleoecology of the Greater Phyllopod Bed community, Burgess Shale. Palaeogeogr. Palaeoclimatol. Palaeoecol. 258, 222–256 (2008).
Caron, J. B., Conway Morris, S. & Shu, D. Tentaculate fossils from the Cambrian of Canada (British Columbia) and China (Yunnan) interpreted as primitive deuterostomes. PLoS ONE 5, e9586 (2010).
Maletz, J. Hemichordata (Enteropneusta & Pterobranchia, incl. Graptolithina): a review of their fossil preservation as organic material. Bull. Geosci. 95, 41–80 (2020).
Kimmig, J., Couto, H., Leibach, W. W. & Lieberman, B. S. Soft-bodied fossils from the upper Valongo Formation (Middle Ordovician: Dapingian-Darriwilian) of northern Portugal. Sci. Nat. 106, 27 (2019).
Botting, J. P., Muir, L. A., Sutton, M. D. & Barnie, T. Welsh gold: a new exceptionally preserved pyritized Ordovician biota. Geology 39, 879–882 (2011).
Fu, D. et al. The Qingjiang biota—a Burgess Shale-type fossil Lagerstätte from the early Cambrian of South China. Science 363, 1338–1342 (2019).
Caron, J. B. & Jackson, D. A. Taphonomy of the Greater Phyllopod Bed community, Burgess Shale. Palaios 21, 451–465 (2006).
Saleh, F. et al. Insights into soft-part preservation from the Early Ordovician Fezouata Biota. Earth Sci. Rev. 213, 103464 (2021).
Zhao, F. et al. Spatial variation in the diversity and composition of the Lower Cambrian (Series 2, Stage 3) Chengjiang biota, Southwest China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 346, 54–65 (2012).
Servais, T., Owen, A. W., Harper, D. A. T., Kröger, B. & Munnecke, A. The Great Ordovician Biodiversification Event (GOBE): the palaeoecological dimension. Palaeogeogr. Palaeoclimatol. Palaeoecol. 294, 99–119 (2010).
Forchielli, A., Steiner, M., Kasbohm, J., Hu, S. & Keupp, H. Taphonomic traits of clay-hosted early Cambrian Burgess Shale-type fossil Lagerstätten in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 398, 59–85 (2014).
Conway Morris, S. Fossil priapulid worms. Spec. Pap. Palaeontol. 20, 1–95 (1977).
Peel, J. S., Stein, M. & Kristensen, R. M. Life cycle and morphology of a Cambrian stem-lineage loriciferan. PLoS ONE 8, e73583 (2013).
Harvey, T. H. & Butterfield, N. J. Exceptionally preserved Cambrian loriciferans and the early animal invasion of the meiobenthos. Nat. Ecol. Evol. 1, 0022 (2017).
Servais, T. et al. The onset of the ‘Ordovician Plankton Revolution’ in the late Cambrian. Palaeogeogr. Palaeoclimatol. Palaeoecol. 458, 12–28 (2016).
Vinn, O. Predation in the Ordovician and Silurian of Baltica. Hist. Biol. 29, 11–16 (2017).
Botting, J. P. Exceptionally well-preserved Middle Ordovician sponges from the Llandegley Rocks Lagerstätte, Wales. Palaeontology 48, 577–617 (2005).
Zhang, L. et al. Diverse cuticular remains in Cambrian (Series 2) SSF assemblages from China and the pioneer metazoan colonization of offshore environments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 567, 110192 (2021).
Harvey, T. H., Ortega-Hernández, J., Lin, J.-P., Zhao, Y.-L. & Butterfield, N. J. Burgess Shale-type microfossils from the middle Cambrian Kaili Formation, Guizhou Province, China. Acta Palaeontol. Pol. 57, 423–436 (2011).
Acknowledgements
The support of the landowners is gratefully acknowledged, as are all contributors to a crowdfunding appeal (including a Holloway Bursary from the Warwickshire Geological Conservation Society, UK) that allowed the purchase of photomicroscope equipment for use by J.P.B. and L.A.M. Reconstruction (Fig. 5) drawn by D.H. Yang, Nanjing Institute of Geology and Palaeontology. L. Parry (University of Oxford, UK) provided helpful comments on some of the material. J.P.B. and L.A.M. are funded by Chinese Academy of Sciences PIFI fellowships (2020VCB0013 and 2018VCB0014, respectively), Y.Z. and J.M. by the National Natural Science Foundation of China (NSFC: 42030510, 41972019) and the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB26000000) and S.P. by a Herchel Smith Postdoctoral Fellowship (University of Cambridge, UK). L.M.E.M. acknowledges the support of Amgueddfa Cymru-National Museum Wales and thanks C. Howells for providing accession numbers for the Castle Bank specimens. B. Slater (Uppsala University, Sweden) advised on interpretation of some SCF material. We thank Y. Fang (NIGPAS, CAS) for assistance with the SEM–EDS analysis and S. Jones (Llandrindod) and G. Steel (Huntington, Herefordshire, UK) for comments on the readability of the paper. This article is a contribution to IGCP project 653 ‘The onset of the Great Ordovician Biodiversification Event’ and to IGCP project 735 ‘Rocks and the rise of Ordovician life: filling knowledge gaps in the Early Palaeozoic biodiversification’.
Author information
Authors and Affiliations
Contributions
This paper was written by J.P.B. and L.A.M., with contributions from all authors. The fauna was discovered and fieldwork conducted by J.P.B. and L.A.M., with interpretation of specimens by J.P.B., L.A.M., S.P. and L.M.E.M. Acid dissolution and analysis/interpretation of SCFs was conducted by E.W. and S.W. Electron microscopy was carried out by S.W. and J.M. Funding was procured by J.M., Y.Z., J.P.B. and L.A.M. The project was conceived and organized by J.P.B. and L.A.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Julien Kimmig, Nigel Hughes and James Schiffbauer for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
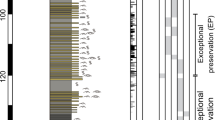
Extended Data Fig. 1 Sedimentological log of the Castle Bank locality, and distribution of exceptional preservation.
a, log of the Castle Bank quarry showing lithological sequence and notes on occurrence of different faunal assemblages; b, ecological log of the two-metre-thick interval of exceptional preservation, showing changing proportions of the graptolite Didymograptus murchisoni to the phosphatic brachiopod Apatobolus micula within the sequence, and correlation with extensive occurrence of soft-tissue preservation (concentrations of graptolites represent generally slower deposition); c, lithological log of the 20-cm-thick interval with Burgess Shale-type preservation, showing higher frequency of event beds (darker grey lenses and layers) that contain Burgess Shale-type preservation; d, polished vertical section through an event bed at level A3, with scale bar 1 mm.
Extended Data Fig. 2 Additional arthropods from the Castle Bank Fauna.
a–d, stem-group xiphosuran from level A0 with detail view of anterior interpretative diagram (b, excluding whip-like posterior that may be an overlain structure), including robust anterior appendages (ap) (enlarged in c), prosoma (shaded, slightly rotated) with eye ridges (ey), mesosoma (meso), segmented metasoma (meta) and base of telson (tel); note also polygonal sculpture on prosoma, visible in d, the counterpart, with second eye ridge arrowed (NMW.2021.3 G.9); e–f, elongate arthropod with cephalocarid-like characters (NMW.2021.3 G.14) with interpretative drawing (based also on additional lighting variations) distinguishing cephalon (ce), thorax with ten segments (th1–10), abdomen with approximately 12 segments (ab1–12) and forked telson (t); g, h, hexapod-like arthropod (as Fig. 1b) with enhanced contrast and interpretative diagram (h), highlighting body regions, three visible limbs (numbered, and in different colours), possible superimposed antenna (ant?) and possible mandible (m?) (NMW.2021.3 G.11); i, j, pair of barnacles, the right attached to the left, with explanatory drawing (ped: peduncle in anterior/posterior view, and broader in lateral view; car: carina; tg: tergum; sc: scutum; ci: cirri) (NMW.2021.3 G.66); k, bivalved arthropod with robust frontal appendages, tail fan and posteriorly spined carapace (NMW.2021.3G.13). Scale bars 1 mm.
Extended Data Fig. 3 Additional new sponge taxa from the Castle Bank fauna.
a, small, complex hexactinellid sponge (level uncertain) with euplectellid-like skeletal strands (NMW.2021.3 G.35); b, tall cylindrical hexactinellid with hypodermal pentactins and rossellid-like architecture (NIGP175889); c, complex conico-cylindrical hexactinellid (NMW.2021.3 G.36); d, stalked ascosponge (new genus) from level A8 with small soft-tissue region (top) supported by long basalia (NMW.2021.3 G.37); e, Teganiella sp. from level A6, the first record from outside the Americas (NMW.2021.3 G.38); f, the most abundant sponge species at the site, a small conical ascosponge from level A9 (NMW.2021.3 G.39i); g, complex hexactinellid (NMW.2021.3 G.40); h, relatively large rossellid-like sponge with abundant prostalia (NMW.2021.3 G.39ii). Scale bars 1 mm.
Extended Data Fig. 4 Deuterostomes from the Castle Bank fauna.
a–c, dendroid graptolite Dictyonema sp. with zooids (NMW.2021.3 G.40); a, cross-polarized light to highlight tubarium; b, plane-polarized light, with dark spots being zooids within thecae; c, detail of zooid (boxed in b) with evidence for cephalic shield (cs) and lophophore (loph) (NMW.2021.3 G.41); d, multi-branched benthic graptolite (NMW.2021.3 G.43); e–g, overall view (with interpretative drawing, f) and detail of body region of hemichordate with lophophore (loph) of two main tentacles and short lateral projections, fusellar-banded tube (tub), and reticulate holdfast region (ho) (NMW.2021.3 G.44); h, pair of Dendrocystites-like solutans from uncertain level (NMW.2021.3 G.45); i, undescribed asterozoan (NMW.2021.3 G.67); j, conodont (NMW.2021.3 G.95); k, bedding plane assemblage of conodonts (NMW.2021.3 G.96). Scale bars 1 mm (except c and k: 0.5 mm).
Extended Data Fig. 5 Vermiform organisms of the Castle Bank Biota.
a–c, unidentified spinose worm with complex jaw apparatus, from level A1, boxed details enlarged in b–c (NMW.2021.3 G.94); b, detail of jaw apparatus; c, anterior margin with tentacles (arrowed); d, e, probable priapulid with narrow cylindrical tube, and extruded soft body (e) (NMW.2021.3 G.97); f–h, archaeopriapulid with complex proboscis (g) showing scalids and multiple zones of pharyngeal teeth (h, labelled A–D) (NMW.2021.3 G.19); i–j, one of several species of kinorhynch-like fossils with proboscis (p), neck (n) with projecting filaments, and eleven plated trunk segments with longitudinal, projecting bristles (NMW.2021.3 G.69); k, palaeoscolecid worm with transverse rows of large plates (NMW.2021.3 G.88); l–m, palaeoscolecid with minute plates, enlarged with cross-polarization in m (NMW.2021.3 G.89). Scale bars 1 mm except for g (0.25 mm), k (0.5 mm) and m (10 μm).
Extended Data Fig. 6 Biomineralized fossils of the Castle Bank fauna.
a, undescribed fenestellid bryozoan from uncertain level, attached to poorly preserved orthocone nautiloid (NMW.2021.3 G.62); b, phosphatic tube of the cnidarian Sphenothallus sp., from uncertain level (NMW.2021.3 G.63); c, brachiopod Monobolina ramsayi from level A0 (NMW.2021.3 G.68); d, unidentified acrotretid brachiopod (NMW.2021.3 G.90); e; encrusting brachiopod Schizocrania multistriata from uncertain level (NMW.2021.3 G.91); f, cnidarian Paraconularia sp. (NMW.2021.3 G.92). Scale bars: a–c, e–f: 1 mm; d: 0.5 mm.
Extended Data Fig. 7 Small carbonaceous fossils from levels A3–4 and A11 at Castle Bank.
a–g, portions of brachiopod shell layers probably sourced from Apatobolus micula; a, PMU 39383/1; b, PMU 39372/1 enlarged in (b1), showing pitted surface produced by spongy wall; c, PMU 39349/3 enlarged in c1, showing pitted microstructure; d, PMU 39387/1 enlarged in (d1) and (d2), showing reticulated to anastomosing networks; e, PMU 39391/1 enlarged in (e1), showing reticulated surface structure; f, PMU 39350/2 enlarged in (f1), showing reticulated patterns grading into anastomosing structures; g–k, variably patterned cuticular fragments; g, PMU 39367/1 enlarged in (g1); h, PMU 39354/1 enlarged in (h1); i, PMU 39387/2; j, PMU 39364/1 enlarged in (j1); k, PMU 39334/1 showing parallel network of elongated pores; l, m, tubular microfossils; l, PMU 39338/1; m, PMU 39375/1; n, PMU 39349/1, graptolite fragment possibly sourced from Didymograptus murchisoni; o, PMU 39350/1, striped cuticular fragment possibly sourced from a wiwaxiid sclerite (see Harvey et al.49, fig. 3); p, PMU 39390/1, scolecodont; q, PMU 39349/2, unknown spine possibly sourced from scolecodonts; r–s, acritarchs; r, PMU 39360/1, sphaeromorph with granulose ornamentation enlarged in (r1); s, PMU 39359/1, Filisphaeridium sp. showing sharp solid processes enlarged in (s1); t–v, chitinozoans; t, PMU 39343/1; u, PMU 39351/1; v, PMU 39363/1 enlarged in (v1) and (v2), showing spinose ornamentation; w, PMU 39381/1, Leiosphaeridia sp. Scale bar equals 50 µm except m (100 µm) and enlargements in a2 (25 µm), a1, d1, d2, e1, f1, g1, h1, j1, r1, s1, v1, v2 (17 µm), b1 and c1 (10 µm).
Extended Data Fig. 8 Current estimated diversity of taxonomic groups in the Castle Bank fauna.
Abundance is given schematically on left side, showing the distribution of different abundance levels (abundant to rare) of the species included within that taxonomic unit. Abundant species are those that are found in almost every block; common species are expected to be found several times in a field day; scarce species may be encountered once in every few field days; and rare species have been found only a small number of times. An absolute scale is provided for taxa known from three, two and one specimens. The diversity figures are estimates pending full taxonomic treatment, especially for sponges and arthropods.
Extended Data Fig. 9 Additional taphonomic data.
a–h, undescribed palaeoscolecid worm (NIGP175890). a, overall SEM view of distal end of specimen; b, elemental map of carbon covering same view; c, high-resolution SEM image of detail of cuticle, showing plate morphology and intervening microplate array; d, detail of distal termination of worm, with elemental maps showing distribution of: e, carbon; f, iron; g, silicon; h, aluminium; i–k, valve of phosphatic (obolid) brachiopod Apatobolus micula (NMW.2021.3 G.64ii) showing preservation as carbon film (j) and virtual absence of phosphate (k); l–p, sponge (as in Extended Data Fig. 3b), with detail of texture of sponge at margin, showing framboid impressions (l), overall view in backscattered SEM (m), and with elemental maps of carbon (m), oxygen (n) and iron (o), showing entirely carbonaceous composition (NIGP175889). Scale bars: a, b, d–l: 100 μm; c: 10 μm; m–p: 1 mm.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Botting, J.P., Muir, L.A., Pates, S. et al. A Middle Ordovician Burgess Shale-type fauna from Castle Bank, Wales (UK). Nat Ecol Evol 7, 666–674 (2023). https://doi.org/10.1038/s41559-023-02038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02038-4
This article is cited by
-
The Cabrières Biota (France) provides insights into Ordovician polar ecosystems
Nature Ecology & Evolution (2024)