Abstract
Evolution can repeat itself, resulting in parallel adaptations in independent lineages occupying similar environments. Moreover, parallel evolution sometimes, but not always, uses the same genes. Two main hypotheses have been put forth to explain the probability and extent of parallel evolution. First, parallel evolution is more likely when shared ecologies result in similar patterns of natural selection in different taxa. Second, parallelism is more likely when genomes are similar because of shared standing variation and similar mutational effects in closely related genomes. Here we combine ecological, genomic, experimental and phenotypic data with Bayesian modelling and randomization tests to quantify the degree of parallelism and its relationship with ecology and genetics. Our results show that the extent to which genomic regions associated with climate are parallel among species of Timema stick insects is shaped collectively by shared ecology and genomic background. Specifically, the extent of genomic parallelism decays with divergence in climatic conditions (that is, habitat or ecological similarity) and genomic similarity. Moreover, we find that climate-associated loci are likely subject to selection in a field experiment, overlap with genetic regions associated with cuticular hydrocarbon traits and are not strongly shaped by introgression between species. Our findings shed light on when evolution is most expected to repeat itself.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The genetic data used in this paper are associated with previous studies and are archived in the National Center for Biotechnology Information (NCBI) Short Read Archive under BioProject Accession Number PRJNA356405. The genome draft 0.3 is available on the Nosil Lab of Evolutionary Biology website (http://nosil-lab.group.shef.ac.uk/?page_id= 25). The climate data and PCA scores used for analyses, a list of accession numbers of sequences used in this study, genotype likelihood files, variant calling format files and associated scripts, input file and scripts for programmes such as Entropy, BayPass and Treemix are available on the DRYAD repository (https://doi.org/10.5061/dryad.51c59zwbr).
Code availability
Computer code is available at https://github.com/karwaan/Timema_climate_adaptation_genomics. All associated data files and scripts for specific analyses are archived on DRYAD (https://doi.org/10.5061/dryad.51c59zwbr). Correspondence for materials (data, scripts or samples) should be addressed to S.C. (samridhi.chaturvedi@gmail.com), Z.G. (zach.gompert@usu.edu) or P.N. (patrik.nosil@cefe.crns.fr).
References
Gould, S. J. Wonderful Life (Radius, 1990).
Blount, Z. D., Borland, C. Z. & Lenski, R. E. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc. Natl Acad. Sci. USA 105, 7899–7906 (2008).
Meyers, P. J. et al. Can the genomics of ecological speciation be predicted across the divergence continuum from host races to species? A case study in Rhagoletis. Phil. Trans. R. Soc. Lond. B 375, 20190534 (2020).
Stern, D. L. & Orgogozo, V. Is genetic evolution predictable? Science 323, 746–751 (2009).
Langerhans, R. B. Predicting evolution with generalized models of divergent selection: a case study with poeciliid fish. Integr. Comp. Biol. 50, 1167–1184 (2010).
Losos, J. B. Convergence, adaptation, and constraint. Evolution 65, 1827–1840 (2011).
Waldvogel, A.-M. et al. Evolutionary genomics can improve prediction of species’ responses to climate change. Evol. Lett. 4, 4–18 (2020).
Lieberman, T. D. et al. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat. Genet. 43, 1275–1280 (2011).
Grant, P. R. & Grant, B. R. Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296, 707–711 (2002).
Bolnick, D. I. et al. (Non)parallel evolution. Annu. Rev. Ecol. Evol. Syst. 49, 303–330 (2018).
Elmer, K. R. & Meyer, A. Adaptation in the age of ecological genomics: insights from parallelism and convergence. Trends Ecol. Evol. 26, 298–306 (2011).
Stern, D. L. The genetic causes of convergent evolution. Nat. Rev. Genet. 14, 751–764 (2013).
Greenway, R. et al. Convergent evolution of conserved mitochondrial pathways underlies repeated adaptation to extreme environments. Proc. Natl Acad. Sci. USA 117, 16424–16430 (2020).
Colosimo, P. F. et al. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science 307, 1928–1933 (2005).
Kingsley, E. P., Manceau, M., Wiley, C. D. & Hoekstra, H. E. Melanism in Peromyscus is caused by independent mutations in Agouti. PloS ONE 4, e6435 (2009).
Manceau, M. et al. Convergence in pigmentation at multiple levels: mutations, genes and function. Phil. Trans. R. Soc. Lond. B 365, 2439–2450 (2010).
Linnen, C. R. et al. Adaptive evolution of multiple traits through multiple mutations at a single gene. Science 339, 1312–1316 (2013).
Barrett, R. D. H. & Schluter, D. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 (2008).
Papadopulos, A. S. T. et al. Rapid parallel adaptation to anthropogenic heavy metal pollution. Mol. Biol. Evol. 38, 3724–3736 (2021).
Yeaman, S. Local adaptation by alleles of small effect. Am. Nat. 186, S74–S89 (2015).
Yeaman, S. et al. Convergent local adaptation to climate in distantly related conifers. Science 353, 1431–1433 (2016).
Chaturvedi, S. et al. The predictability of genomic changes underlying a recent host shift in Melissa blue butterflies. Mol. Ecol. 27, 2651–2666 (2018).
Arendt, J. & Reznick, D. Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation? Trends Ecol. Evol. 23, 26–32 (2008).
Conte, G. L., Arnegard, M. E., Peichel, C. L. & Schluter, D. The probability of genetic parallelism and convergence in natural populations. Proc. Biol. Sci. 279, 5039–5047 (2012).
Bailey, S. F., Rodrigue, N. & Kassen, R. The effect of selection environment on the probability of parallel evolution. Mol. Biol. Evol. 32, 1436–1448 (2015).
Lenski, R. E. Experimental evolution and the dynamics of adaptation and genome evolution in microbial populations. ISME J. 11, 2181–2194 (2017).
Roda, F. et al. Convergence and divergence during the adaptation to similar environments by an Australian groundsel. Evolution 67, 2515–2529 (2013).
Stuart, Y. E. et al. Contrasting effects of environment and genetics generate a continuum of parallel evolution. Nat. Ecol. Evol. 1, 158 (2017).
Morales, H. E. et al. Genomic architecture of parallel ecological divergence: beyond a single environmental contrast. Sci. Adv. 5, eaav9963 (2019).
Manousaki, T. et al. Parsing parallel evolution: ecological divergence and differential gene expression in the adaptive radiations of thick-lipped Midas cichlid fishes from Nicaragua. Mol. Ecol. 22, 650–669 (2013).
Rennison, D. J. Shared patterns of genome-wide differentiation are more strongly predicted by geography than by ecology. Am. Nat. 195, 192–200 (2020).
Schluter, D., Clifford, E. A., Nemethy, M. & McKinnon, J. S. Parallel evolution and inheritance of quantitative traits. Am. Nat. 163, 809–822 (2004).
Roesti, M., Gavrilets, S., Hendry, A. P., Salzburger, W. & Berner, D. The genomic signature of parallel adaptation from shared genetic variation. Mol. Ecol. 23, 3944–3956 (2014).
Meyers, P. J. et al. Can the genomics of ecological speciation be predicted across the divergence continuum from host races to species? A case study in Rhagoletis. Phil. Trans. R. Soc. B 375, 20190534 (2020).
Matos, M. et al. History, chance and selection during phenotypic and genomic experimental evolution: replaying the tape of life at different levels. Front. Genet. 6, 71 (2015).
Good, B. H. et al. The dynamics of molecular evolution over 60,000 generations. Nature 551, 45–50 (2017).
Storz, J. F. Causes of molecular convergence and parallelism in protein evolution. Nat. Rev. Genet. 17, 239–250 (2016).
Kohler, A. et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat. Genet. 47, 410–415 (2015).
Haldane, J. B. The Causes of Evolution (Princeton Univ. Press, 1990).
Gompel, N. & Carroll, S. B. Genetic mechanisms and constraints governing the evolution of correlated traits in drosophilid flies. Nature 424, 931–935 (2003).
Orgogozo, V. Replaying the tape of life in the twenty-first century. Interface Focus 5, 20150057 (2015).
Blount, Z. D., Lenski, R. E. & Losos, J. B. Contingency and determinism in evolution: replaying life’s tape. Science 362, eaam5979 (2018).
Nosil, P. Divergent host plant adaptation and reproductive isolation between ecotypes of Timema cristinae walking sticks. Am. Nat. 169, 151–162 (2007).
Comeault, A. A., Carvalho, C. F., Dennis, S., Soria-Carrasco, V. & Nosil, P. Color phenotypes are under similar genetic control in two distantly related species of Timema stick insect. Evolution 70, 1283–1296 (2016).
Comeault, A. A. et al. Selection on a genetic polymorphism counteracts ecological speciation in a stick insect. Curr. Biol. 25, 1975–1981 (2015).
Lindtke, D. et al. Long-term balancing selection on chromosomal variants associated with crypsis in a stick insect. Mol. Ecol. 26, 6189–6205 (2017).
Villoutreix, R. et al. Large-scale mutation in the evolution of a gene complex for cryptic coloration. Science 369, 460–466 (2020).
Barghi, N., Hermisson, J. & Schlötterer, C. Polygenic adaptation: a unifying framework to understand positive selection. Nat. Rev. Genet. 21, 769–781 (2020).
Rockman, M. V. The QTN program and the alleles that matter for evolution: all that’s gold does not glitter. Evolution 66, 1–17 (2012).
Law, J. H. & Crespi, B. J. The evolution of geographic parthenogenesis in Timema walking-sticks. Mol. Ecol. 11, 1471–1489 (2002).
Nosil, P. et al. Ecology shapes epistasis in a genotype-phenotype-fitness map for stick insect colour. Nat. Ecol. Evol. 4, 1673–1684 (2020).
Siepielski, A. M. et al. Precipitation drives global variation in natural selection. Science 355, 959–962 (2017).
De La Torre, A., R., Wilhite, B., W. & Neale, D. B. Environmental genome-wide association reveals climate adaptation is shaped by subtle to moderate allele frequency shifts in loblolly pine. Genome Biol. Evol. 11, 2976–2989 (2019).
Nosil, P. et al. Natural selection and the predictability of evolution in Timema stick insects. Science https://science.sciencemag.org/content/359/6377/765 (2018).
Riesch, R. et al. Transitions between phases of genomic differentiation during stick-insect speciation. Nat. Ecol. Evol. 1, 82 (2017).
Harvey, M. G. et al. Sequence capture versus restriction site associated DNA sequencing for shallow systematics. Syst. Biol. 65, 910–924 (2016).
Schluter, D. & Conte, G. L. Genetics and ecological speciation. Proc. Natl Acad. Sci. USA 106, 9955–9962 (2009).
Liu, S., Ferchaud, A. ‐L., Grønkjær, P., Nygaard, R. & Hansen, M. M. Genomic parallelism and lack thereof in contrasting systems of three‐spined sticklebacks. Mol. Ecol. 27, 4725–4743 (2018).
Gompert, Z. et al. Admixture and the organization of genetic diversity in a butterfly species complex revealed through common and rare genetic variants. Mol. Ecol. 23, 4555–4573 (2014).
Spiegelhalter, D. J., Best, N. G., Carlin, B. P. & Van Der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. B 64, 583–639 (2002).
Gompert, Z. et al. Experimental evidence for ecological selection on genome variation in the wild. Ecol. Lett. 17, 369–379 (2014).
Sprenger, P. P., Lars, H. B., Abou, B., Federle, W. & Menzel, F. Coping with the climate: cuticular hydrocarbon acclimation of ants under constant and fluctuating conditions. J. Exp. Biol. 221, jeb171488 (2018).
Botella-Cruz, M., Velasco, J., Millán, A., Hetz, S. & Pallarés, S. Cuticle hydrocarbons show plastic variation under desiccation in saline aquatic beetles. Insects 12, 285 (2021).
Walden, N., Lucek, K. & Willi, Y. Lineage-specific adaptation to climate involves flowering time in North American Arabidopsis lyrata. Mol. Ecol. 29, 1436–1451 (2020).
Rose, N. H., Bay, R. A., Morikawa, M. K. & Palumbi, S. R. Polygenic evolution drives species divergence and climate adaptation in corals. Evolution 72, 82–94 (2018).
Blanco-Pastor, J. L. et al. Annual and perennial Medicago show signatures of parallel adaptation to climate and soil in highly conserved genes. Mol. Ecol. https://doi.org/10.1111/mec.16061 (2021).
Wang, L. et al. Molecular parallelism underlies convergent highland adaptation of maize landraces. Mol. Biol. Evol. 38, 3567–3580 (2021).
Bohutínská, M. et al. Genomic basis of parallel adaptation varies with divergence in Arabidopsis and its relatives. Proc. Natl Acad. Sci. USA 118, e2022713118 (2021).
Lobréaux, S. & Melodelima, C. Detection of genomic loci associated with environmental variables using generalized linear mixed models. Genomics 105, 69–75 (2015).
Frachon, L. et al. A genomic map of climate adaptation in Arabidopsis thaliana at a micro-geographic scale. Front. Plant Sci. 9, 967 (2018).
Contreras-Moreira, B. et al. Genetic association with high-resolution climate data reveals selection footprints in the genomes of barley landraces across the Iberian Peninsula. Mol. Ecol. 28, 1994–2012 (2019).
Heliconius Genome Consortium. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487, 94–98 (2012).
Henning, F. & Meyer, A. The evolutionary genomics of cichlid fishes: explosive speciation and adaptation in the postgenomic era. Annu. Rev. Genomics Hum. Genet. 15, 417–441 (2014).
Marburger, S. et al. Interspecific introgression mediates adaptation to whole genome duplication. Nat. Commun. 10, 5218 (2019).
Giska, I. et al. Introgression drives repeated evolution of winter coat color polymorphism in hares. Proc. Natl Acad. Sci. USA 116, 24150–24156 (2019).
Menon, M. et al. Adaptive evolution in a conifer hybrid zone is driven by a mosaic of recently introgressed and background genetic variants. Commun. Biol. 4, 160 (2021).
Bay, R. A., Taylor, E. B. & Schluter, D. Parallel introgression and selection on introduced alleles in a native species. Mol. Ecol. 28, 2802–2813 (2019).
Zhang, X., Ryaner, J. G., Blaxter, M. & Bailey, N. W. Rapid parallel adaptation despite gene flow in silent crickets. Nat. Commun. 12, 50 (2021).
Marques, D. A., Jones, F. C., Di Palma, F., Kingsley, D. M. & Reimchen, E. Experimental evidence for rapid genomic adaptation to a new niche in an adaptive radiation. Nat. Ecol. Evol. 2, 1128–1138 (2018).
Li, H. et al. and 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. GigaScience https://doi.org/10.1093/gigascience/giab008 (2021).
Gautier, M. Genome-wide scan for adaptive divergence and association with population-specific covariates. Genetics 201, 1555–1579 (2015).
Günther, T. & Coop, G. Robust identification of local adaptation from allele frequencies. Genetics 195, 205–220 (2013).
Clarke, R. T., Rothery, P. & Raybould, A. F. Confidence limits for regression relationships between distance matrices: estimating gene flow with distance. J. Agric. Biol. Environ. Stat. 7, 361–372 (2002).
Soria-Carrasco, V. et al. Stick insect genomes reveal natural selection’s role in parallel speciation. Science 344, 738–742 (2014).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria (2022).
Acknowledgements
S.C. was supported by the Utah State University College of Science and School of Graduate Studies. M.M. was supported by Swiss National Science Foundation Postdoc Mobility grants PBBSP3_141367 and P300P3_147888. Z.G. was supported by the US NSF (DEB 1844941). This study is part of a project that has received funding from the European Research Council (ERC) to P.N., under the European Union’s Horizon 2020 research and innovation programme (Grant agreement No. 770826 EE-Dynamics). J.L.F. was supported by NSF Dimensions in Biodiversity grant 1638997. The support and resources from the Center for High-Performance Computing at the University of Utah are gratefully acknowledged. We thank Landells-Hill Big Creek Reserve (https://doi.org/10.21973/N3NH24) where part of the samples included in this study were collected.
Author information
Authors and Affiliations
Contributions
P.N., M.M., O.G.O., Z.G., J.L.F. and S.C. designed the study. P.N., M.M., R.R. and V.S.-C. collected data. Z.G., O.G.O. and S.C. analysed the data. S.C. wrote the manuscript with feedback from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Megan Ruffley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
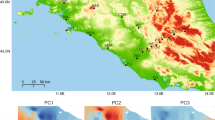
Extended Data Fig. 1 PCA of climate variation.
Ordination of climate variation (22 variables, see Supplementary Table 2 for code descriptions) via principal component analysis (PCA). Points denote the study populations, colour-coded by species.
Extended Data Fig. 2 Manhattan plot for PC1.
Manhattan plots showing the strength of evidence for association (measured here using the Bayes factor from the software BayPass) between a SNP window and climate for PC1. Results are shown along the 13 linkage groups. In each panel title, the two values in parentheses are the number of SNP windows in the top 10% quantile (‘windows’), followed by the number of linkage groups with at least 1 SNP window in the top 10% quantile (‘LG’).
Extended Data Fig. 3 Manhattan plot for PC2.
Manhattan plots showing the strength of evidence for association (measured here using the Bayes factor from the software BayPass) between a SNP window and climate for PC2. Results are shown along the 13 linkage groups. In each panel title, the two values in parentheses are the number of SNP windows in the top 10% quantile (‘windows’), followed by the number of linkage groups with at least 1 SNP window in the top 10% quantile (‘LG’).
Extended Data Fig. 4 Parallelism tests for PC1.
Tests for parallel climate-associated SNP windows between species of Timema stick insects (all plots are for the top 10% empirical quantile) for PC1. Plot shows x-fold enrichments for the number of overlapping climate-associated SNP windows for PC1 for comparisons between multiple species, that is, beyond pairs of species (for example, 2 or more species, 3 or more species, 4 or more species). Gray dots denote x-fold values expected under 1000 randomizations for a null distribution. Black diamond denotes median of the x-fold values expected under 1000 randomizations for a null distribution. Red dot and N value above each group indicates the observed number of overlapping climate-associated SNP windows for each comparison. P-value above each group denotes whether the overlap is greater than expected by chance from a one-sided randomization test. * Indicates x-fold enrichments with P-value ≤ 0.05.
Extended Data Fig. 5 Parallelism tests for PC2.
Tests for parallel climate-associated SNP windows between species of Timema stick insects (all plots are for the top 10% empirical quantile) for PC2. Plot shows x-fold enrichments for the number of overlapping climate-associated SNP windows for PC2 for comparisons between multiple species, that is, beyond pairs of species (for example, 2 or more species, 3 or more species, 4 or more species). Gray dots denote x-fold values expected under 1000 randomizations for a null distribution. Black diamond denotes median of the x-fold values expected under 1000 randomizations for a null distribution. Red dot and N value above each group indicates the observed number of overlapping climate-associated SNP windows for each comparison. P-value above each group denotes whether the overlap is greater than expected by chance from a one-sided randomization test. * Indicates x-fold enrichments with P-value ≤ 0.05.
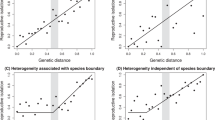
Extended Data Fig. 6 Tests of the ‘shared ecology’ versus ‘shared genetics’ hypothesis for PC1.
Test results of the ‘shared ecology’ versus ‘shared genetics’ hypotheses for PC1. (a) Scatterplot shows the relationship between X-fold enrichment (measure for parallelism) and climatic distance (measured as the distance in PC1 scores) based on a single-factor linear model. (b) Scatterplot shows the relationship between X-fold enrichment (measure for parallelism) and genetic distance (measured as pairwise phylogenetic distance) based on a single-factor linear model. (c) Scatterplot shows the relationship between climatic distance (measured as the distance in PC1 scores and is the distance in climate variables) and genetic distance (calculated as pairwise phylogenetic distance) based on a single-factor linear model. (d) Plot shows parameter estimates with standardized coefficients for the full model for PC1. This test was implemented for all eight species and 56 species pairs. Error bars indicate 95% equal-tail probability intervals (ETPIs). A negative or positive estimate that deviates from zero indicates the effect on parallelism.
Extended Data Fig. 7 Tests of the ‘shared ecology’ versus ‘shared genetics’ hypothesis for PC2.
Test results of the ‘shared ecology’ versus ‘shared genetics’ hypotheses for PC2. (a) Scatterplot shows the relationship between X-fold enrichment (measure for parallelism) and climatic distance (measured as the distance in PC2 scores) based on a single-factor linear model. (b) Scatterplot shows the relationship between X-fold enrichment (measure for parallelism) and genetic distance (measured as pairwise phylogenetic distance) based on a single-factor linear model. (c) Scatterplot shows the relationship between climatic distance (measured as the distance in PC2 scores and is the distance in climate variables) and genetic distance (calculated as pairwise phylogenetic distance) based on a single-factor linear model. (d) Plot shows parameter estimates with standardized coefficients for the full model only for PC2. This test was implemented for all eight species and 56 species pairs. Error bars indicate 95% equal-tail probability intervals (ETPIs). A negative or positive estimate that deviates from zero indicates the effect on parallelism.
Supplementary information
Supplementary Information
Supplementary methods, results, references, tables and figures.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaturvedi, S., Gompert, Z., Feder, J.L. et al. Climatic similarity and genomic background shape the extent of parallel adaptation in Timema stick insects. Nat Ecol Evol 6, 1952–1964 (2022). https://doi.org/10.1038/s41559-022-01909-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01909-6
This article is cited by
-
Divergent dynamics of sexual and habitat isolation at the transition between stick insect populations and species
Nature Communications (2024)