Abstract
Tropical forests are some of the most biodiverse ecosystems in the world, yet their functioning is threatened by anthropogenic disturbances and climate change. Global actions to conserve tropical forests could be enhanced by having local knowledge on the forestsʼ functional diversity and functional redundancy as proxies for their capacity to respond to global environmental change. Here we create estimates of plant functional diversity and redundancy across the tropics by combining a dataset of 16 morphological, chemical and photosynthetic plant traits sampled from 2,461 individual trees from 74 sites distributed across four continents together with local climate data for the past half century. Our findings suggest a strong link between climate and functional diversity and redundancy with the three trait groups responding similarly across the tropics and climate gradient. We show that drier tropical forests are overall less functionally diverse than wetter forests and that functional redundancy declines with increasing soil water and vapour pressure deficits. Areas with high functional diversity and high functional redundancy tend to better maintain ecosystem functioning, such as aboveground biomass, after extreme weather events. Our predictions suggest that the lower functional diversity and lower functional redundancy of drier tropical forests, in comparison with wetter forests, may leave them more at risk of shifting towards alternative states in face of further declines in water availability across tropical regions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The vegetation census and plant functional traits data that support the findings of this study are available from their sources (www.ForestPlots.net and gem.tropicalforests.ox.ac.uk/). To comply with the original data owners, the processed community-level data used in this study can be accessed through the corresponding author upon request.
Code availability
All relevant R functions and code used in this study are referred to in the Methods section and can be accessed at https://doi.org/10.5281/zenodo.6367982.
References
Barlow, J. et al. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535, 144–147 (2016).
Beech, E., Rivers, M., Oldfield, S. & Smith, P. P. GlobalTreeSearch: the first complete global database of tree species and country distributions. J. Sustain. 36, 454–489 (2017).
ter Steege, H. et al. The discovery of the Amazonian tree flora with an updated checklist of all known tree taxa. Sci. Rep. 6, 29549 (2016).
Hubau, W. et al. Asynchronous carbon sink saturation in African and Amazonian tropical forests. Nature 579, 80–87 (2020).
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Maia, V. A. et al. The carbon sink of tropical seasonal forests in southeastern Brazil can be under threat. Sci. Adv. 6, eabd4548 (2020).
Malhi, Y. et al. The regional variation of aboveground live biomass in old‐growth Amazonian forests. Glob. Change Biol. 12, 1107–1138 (2006).
Phillips, O. L. et al. Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347 (2009).
Malhi, Y. et al. Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172 (2008).
Gatti, L. V. et al. Amazonia as a carbon source linked to deforestation and climate change. Nature 595, 388–393 (2021).
Hisano, M., Searle, E. B. & Chen, H. Y. Biodiversity as a solution to mitigate climate change impacts on the functioning of forest ecosystems. Biol. Rev. 93, 439–456 (2018).
Pecl, G. T. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214 (2017).
Malhi, Y. et al. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl Acad. Sci. USA 106, 20610–20615 (2009).
Seager, R. et al. Climatology, variability, and trends in the US vapor pressure deficit, an important fire-related meteorological quantity. J. Appl. Meteorol. Climatol. 54, 1121–1141 (2015).
Smith, M. N. et al. Empirical evidence for resilience of tropical forest photosynthesis in a warmer world. Nat. Plants 6, 1225–1230 (2020).
Yuan, W. et al. Increased atmospheric vapor pressure deficit reduces global vegetation growth. Sci. Adv. 5, eaax1396 (2019).
Costa, F. R. C., Schietti, J., Stark, S. C. & Smith, M. N. The other side of tropical forest drought: do shallow water table regions of Amazonia act as large‐scale hydrological refugia from drought?. New Phytol. https://doi.org/10.1111/nph.17914 (2022).
Brodribb, T. J., Powers, J., Cochard, H. & Choat, B. Hanging by a thread? Forests and drought. Science 368, 261–266 (2020).
Allen, K. et al. Will seasonally dry tropical forests be sensitive or resistant to future changes in rainfall regimes? Environ. Res. Lett. 12, 023001 (2017).
Esquivel‐Muelbert, A. et al. Compositional response of Amazon forests to climate change. Glob. Change Biol. 25, 39–56 (2019).
Aguirre‐Gutiérrez, J. et al. Drier tropical forests are susceptible to functional changes in response to a long‐term drought. Ecol. Lett. 22, 855–865 (2019).
Cadotte, M. W., Carscadden, K. & Mirotchnick, N. Beyond species: functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48.5, 1079–1087 (2011).
Aguirre‐Gutiérrez, J. et al. Butterflies show different functional and species diversity in relationship to vegetation structure and land use. Glob. Ecol. Biogeogr. 26, 1126–1137 (2017).
Arruda Almeida, B. et al. Comparing species richness, functional diversity and functional composition of waterbird communities along environmental gradients in the neotropics. PLoS ONE 13, e0200959 (2018).
Yachi, S. & Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468 (1999).
Correia, D. L. P., Raulier, F., Bouchard, M. & Filotas, É. Response diversity, functional redundancy, and post‐logging productivity in northern temperate and boreal forests. Ecol. Appl. 28, 1282–1291 (2018).
Elmqvist, T. et al. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 1, 488–494 (2003).
Loreau, M. & de Mazancourt, C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol. Lett. 16, 106–115 (2013).
Petchey, O. L., Evans, K. L., Fishburn, I. S. & Gaston, K. J. Low functional diversity and no redundancy in British avian assemblages. J. Anim. Ecol. 76, 977–985 (2007).
Jucker, T. et al. Stabilizing effects of diversity on aboveground wood production in forest ecosystems: linking patterns and processes. Ecol. Lett. 17, 1560–1569 (2014).
Fonseca, C. R. & Ganade, G. Species functional redundancy, random extinctions and the stability of ecosystems. J. Ecol. 89, 118–125 (2001).
Aguirre-Gutiérrez, J. et al. Long-term droughts may drive drier tropical forests towards increased functional, taxonomic and phylogenetic homogeneity. Nat. Commun. 11, 3346 (2020).
Fauset, S. et al. Drought‐induced shifts in the floristic and functional composition of tropical forests in Ghana. Ecol. Lett. 15, 1120–1129 (2012).
Laliberté, E. & Legendre, P. A distance‐based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010).
Bauman, D. et al. Tropical tree growth sensitivity to climate is driven by species intrinsic growth rate and leaf traits. Glob. Change Biol. 28, 1414–1432 (2022).
Quesada, C. et al. Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 9, 2203–2246 (2012).
Bennett, A. C. et al. Resistance of African tropical forests to an extreme climate anomaly. Proc. Natl Acad. Sci. USA 118, e2003169118 (2021).
Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems (eds Shukla, P.R. et al.) (IPCC, 2019).
Ashton, I. W., Miller, A. E., Bowman, W. D. & Suding, K. N. Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology 91, 3252–3260 (2010).
Petchey, O. L. On the statistical significance of functional diversity effects. Funct. Ecol. 18, 297–303 (2004).
Bruno, J. F., Stachowicz, J. J. & Bertness, M. D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125 (2003).
ter Steege, H. et al. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443, 444–447 (2006).
Raes, N. et al. Botanical richness and endemicity patterns of Borneo derived from species distribution models. Ecography 32, 180–192 (2009).
Shenkin, A. et al. The influence of ecosystem and phylogeny on tropical tree crown size and shape. Front. For. Glob. Change 3, 501757 (2020).
Harrison, S., Spasojevic, M. J. & Li, D. Climate and plant community diversity in space and time. Proc. Natl Acad. Sci. USA 117, 4464–4470 (2020).
Grossman, J. J., Cavender‐Bares, J., Hobbie, S. E., Reich, P. B. & Montgomery, R. A. Species richness and traits predict overyielding in stem growth in an early‐successional tree diversity experiment. Ecology 98, 2601–2614 (2017).
Williams, L. J. et al. Remote spectral detection of biodiversity effects on forest biomass. Nat. Ecol. Evol. 5, 46–54 (2021).
Hutchison, C., Gravel, D., Guichard, F. & Potvin, C. Effect of diversity on growth, mortality, and loss of resilience to extreme climate events in a tropical planted forest experiment. Sci. Rep. 8, 15443 (2018).
González-M, R. et al. Diverging functional strategies but high sensitivity to an extreme drought in tropical dry forests. Ecol. Lett. 24, 451–463 (2021).
Hoegh-Guldberg, O. et al. in IPCC Special Report on Global Warming of 1.5 °C (eds Masson-Delmotte, V. et al.) Ch. 3 (WMO, 2018).
de la Riva, E. G. et al. The importance of functional diversity in the stability of Mediterranean shrubland communities after the impact of extreme climatic events. J. Plant Ecol. 10, 281–293 (2017).
Reich, P. B. The world-wide “fast-slow” plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301 (2014).
Oliveira, R. S. et al. Linking plant hydraulics and the fast–slow continuum to understand resilience to drought in tropical ecosystems. New Phytol. 230, 904–923 (2021).
Anderegg, W. R. L. & Meinzer, F. C. in Functional and Ecological Xylem Anatomy (ed Hacke, U.) Ch. 9 (Springer, 2015).
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009).
Pratt, R., Jacobsen, A., Ewers, F. & Davis, S. Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phytol. 174, 787–798 (2007).
Zanne, A. E. et al. Angiosperm wood structure: global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 97, 207–215 (2010).
Bucci, S. J. et al. The stem xylem of Patagonian shrubs operates far from the point of catastrophic dysfunction and is additionally protected from drought‐induced embolism by leaves and roots. Plant Cell Environ. 36, 2163–2174 (2013).
Meinzer, F. C. et al. Coordination of leaf and stem water transport properties in tropical forest trees. Oecologia 156, 31–41 (2008).
Scholz F. G., Phillips N. G., Bucci S. J., Meinzer F. C. & Goldstein G. in Size- and Age-Related Changes in Tree Structure and Function (eds Meinzer F. C. C. et al.) 341–361 (Springer, 2011).
Mitchell, P. J. et al. Using multiple trait associations to define hydraulic functional types in plant communities of south-western Australia. Oecologia 158, 385–397 (2008).
Villagra, Mariana et al. Functional relationships between leaf hydraulics and leaf economic traits in response to nutrient addition in subtropical tree species. Tree Physiol. 33, 1308–1318 (2013).
Ishida, Atsushi et al. Coordination between leaf and stem traits related to leaf carbon gain and hydraulics across 32 drought-tolerant angiosperms. Oecologia 156, 193–202 (2008).
Malhi, Y. et al. The Global Ecosystems Monitoring network: monitoring ecosystem productivity and carbon cycling across the tropics. Biol. Conserv. 253, 108889 (2021).
Martin, R. E. et al. Covariance of sun and shade leaf traits along a tropical forest elevation gradient. Front. Plant Sci. 10, 1810 (2020).
Enquist, B. J. et al. Assessing trait‐based scaling theory in tropical forests spanning a broad temperature gradient. Glob. Ecol. Biogeogr. 26, 1357–1373 (2017).
Both, S. et al. Logging and soil nutrients independently explain plant trait expression in tropical forests. New Phytol. 221, 1853–1865 (2019).
Oliveras, I. et al. The influence of taxonomy and environment on leaf trait variation along tropical abiotic gradients. Front. For. Glob. Change https://doi.org/10.3389/ffgc.2020.00018 (2020).
Gvozdevaite, A. et al. Leaf-level photosynthetic capacity dynamics in relation to soil and foliar nutrients along forest–savanna boundaries in Ghana and Brazil. Tree Physiol. 38, 1912–1925 (2018).
Aguirre-Gutiérrez, J. et al. Pantropical modelling of canopy functional traits using Sentinel-2 remote sensing data. Remote Sens. Environ. 252, 112122 (2021).
Pavoine, S. adiv: an R package to analyse biodiversity in ecology. Methods Ecol. Evol. 11, 1106–1112 (2020).
Pavoine, S. & Ricotta, C. A simple translation from indices of species diversity to indices of phylogenetic diversity. Ecol. Ind. 101, 552–561 (2019).
Ricotta, C. et al. Measuring the functional redundancy of biological communities: a quantitative guide. Methods Ecol. Evol. 7, 1386–1395 (2016).
Díaz, S. et al. The global spectrum of plant form and function. Nature 529, 167–171 (2016).
van der Plas, F., Van Klink, R., Manning, P., Olff, H. & Fischer, M. Sensitivity of functional diversity metrics to sampling intensity. Methods Ecol. Evol. 8, 1072–1080 (2017).
Rao, C. R. Diversity and dissimilarity coefficients: a unified approach. Theor. Popul. Biol. 21, 24–43 (1982).
Simpson, E. H. Measurement of diversity. Nature https://doi.org/10.1038/163688a0 (1949).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Abatzoglou, J. T., Dobrowski, S. Z., Parks, S. A. & Hegewisch, K. C. TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 5, 170191 (2018).
Fan, Y. Groundwater in the earth’s critical zone: relevance to large-scale patterns and processes. Water Resour. Res. 51, 3052–3069 (2015).
Moulatlet, G. M. et al. Using digital soil maps to infer edaphic affinities of plant species in Amazonia: problems and prospects. Ecol. Evol. 7, 8463–8477 (2017).
Dormann, C. F. et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46 (2013).
Vehtari, A., Gelman, A. & Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27, 1413–1432 (2017).
Makowski, D., Ben-Shachar, M. S. & Lüdecke, D. bayestestR: Describing effects and their uncertainty, existence and significance within the Bayesian framework. J. Open Source Softw. 4, 1541 (2019).
Kruschke, J. K. Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan (Academic Press, 2014).
Acknowledgements
This work is a product of the Global Ecosystems Monitoring (GEM) network (gem.tropicalforests.ox.ac.uk). J.A.-G. was funded by the Natural Environment Research Council (NERC; NE/T011084/1) and the Oxford University Jhon Fell Fund (10667). The traits field campaign was funded by a grant to Y.M. from the European Research Council (advanced grant GEM-TRAIT: 321131) under the European Union’s Seventh Framework Programme (FP7/2007–2013) with additional support from NERC grant NE/D014174/1 and NE/J022616/1 for traits work in Peru, NERC grant ECOFOR (NE/K016385/1) for traits work in Santarem, NERC grant BALI (NE/K016369/1) for plot and traits work in Malaysia and ERC advanced grant T-FORCES (291585) to O.L.P. for traits work in Australia. Plot setup in Ghana and Gabon was funded by a NERC grant NE/I014705/1 and by the Royal Society-Leverhulme Africa Capacity Building Programme. The Malaysia campaign was also funded by NERC grant NE/K016253/1. Plot inventories in Peru were supported by funding from the US National Science Foundation Long-Term Research in Environmental Biology program (LTREB; DEB 1754647) and the Gordon and Betty Moore Foundation Andes–Amazon Program. Plots inventories in Nova Xavantina (Brazil) were supported by the National Council for Scientific and Technological Development (CNPq), Long Term Ecological Research Program (PELD), process 441244/2016–5 and the Foundation of Research Support of Mato Grosso (FAPEMAT), Project ReFlor, process 589267/2016. During data collection, I.O.M. was supported by a Marie Curie Fellowship (FP7-PEOPLE-2012-IEF-327990). GEM trait data in Gabon were supported by the Gabon National Parks Agency. D.B. was funded by the Belgian American Educational Foundation (BAEF) and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement number 895799. W.D.K. acknowledges funding from the University of Amsterdam via a starting grant and through the Faculty Research Cluster ‘Global Ecology’. S.A.-B. acknowledges funding from The Leverhulme Trust—Royal Society of the United Kingdom (A130026) under the Water Stress, Ecosystem Function and tree FD in tropical African forests project. C.A.J. acknowledges support from the Brazilian National Research Council/CNPq (PELD process 403710/2012–0), NERC and the State of São Paulo Research Foundation/FAPESP as part of the projects Functional Gradient, PELD/BIOTA and ECOFOR (processes 2003/12595-7, 2012/51509-8 and 2012/51872-5, within the BIOTA/FAPESP Program—The Biodiversity Virtual Institute (www.biota.org.br); COTEC/IF 002.766/2013 and 010.631/2013 permits. B.S.M. was supported by the CNPq/PELD projects (number 441244/2016-5 and number 441572/2020-0) and CAPES (number 136277/2017-0). D.F.R.P.B. thanks the financial support from NERC (NE/K016253/1) for trait data collection in Sabah Malaysia. M.S. acknowledges funding for Andes Biodiversity and Ecosystem Research Group (ABERG) plot network from the US National Science Foundation (NSF) Long-Term Research in Environmental Biology (LTREB) 1754647, the Gordon and Betty Moore Foundation’s Andes to Amazon Initiative and RAINFOR. E.B, J.B. and Y.M. acknowledge the support from NERC under projects NE/K016431/1 and NE/S01084X/1. R.M.E. acknowledges support from the Sime Darby Foundation. Measurements and analysis include support from NERC (‘AMAZONICAʼ, NE/F005806; ‘BIO-REDʼ, NE/N012542/1; ARBOLES, NE/S011811/1), the Moore Foundation and the AfriTRON and RAINFOR networks. Y.M. is supported by the Jackson Foundation.
Author information
Authors and Affiliations
Contributions
J.A.-G. conceived the study, designed and carried out the analysis and wrote the first draft of the paper. E.B. contributed to the main ideas and design of the study. Y.M. conceived and implemented the GEM Network, obtained funding for most of the GEM traits field campaigns and commented on earlier versions of the manuscript. J.A.-G., E.B., I.O.M., D.B., J.J.C.-R., M.G.N.-M., S.B., J.E.N., F.E.O., N.N.B., V.M., J.W.D., K.H., A.F., R.G.-M., N.N., A.B.H.-M., D.G., B.S.-N., S.M.R., M.M.M.S., W.F.-R., A.S., T.R., C.A.J.G., S.M., K.A., G.P.A., L.P.B., D.F.R.P.B., L.A.C., B.J.E., R.M.E., J.F., K.J.J., C.A.J., B.H.M.-J., R.E.M., P.S.M., O.L.P., A.C.B., S.L.L., C.A.Q., B.S.M., W.D.K., M.S., Y.A.T., L.J.T.W., N.S., D.A.C., J.B., S.A.-B. and Y.M. participated in or coordinated vegetation, trait data and/or soil data collection or processed field data and commented on and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Vinícius Andrade Maia, Flávia Costa, Rebecca Ostertag and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Violin plots of the plant functional traits and their value ranges across the study area.
The plant functional traits used in the study with raw trait values are shown (corresponding to n = 2461 individual trees) but these data were log-transformed prior to further analysis. The colours correspond to the field sampling areas where in situ traits collection plots are located; the Y axis shows the raw data values for each functional trait. Photosynthetic traits are Amax: Light-saturated maximum rates of net photosynthesis at saturated CO2 (2000 ppm CO2); μmol m-2 s-1), Asat: light-saturated rates of net photosynthesis at ambient CO2 concentration (400 ppm CO2; μmol m−2 s−1), RDark: dark respiration (μmol m−2 s−1). Leaf nutrient concentration traits are, Ca: leaf calcium (%), K: leaf potassium (%), Mg: leaf magnesium (%), N: leaf nitrogen (%), P: leaf phosphorus (%). Leaf morphological and structural traits are, A: leaf area (cm²), DM: leaf dry mass (g), FM: leaf fresh mass (g), LDMC: leaf dry matter content (mg/g), LWC: leaf water content (%), SLA: specific leaf area (m2/g), T: leaf thickness (mm), WD: wood density (g/cm3). No traits were collected in Mexico and were thus assigned to the vegetation censuses from other locations as explained in the methods section. Brazil -ST: Brazil Santarem, Brazil -NX: Brazil Nova Xavantina. The horizontal lines within each boxplot represent the mean trait value and the vertical lines encompass the first (25th) and third (75th) quartiles of the data distribution for each trait.
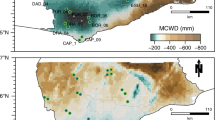
Extended Data Fig. 2 Density plots of the climatic and soil conditions that encompass each field sampling location where plant functional traits and vegetation censuses were collected.
The top density graph of each climatic and soil variable shows the values found across the tropical and subtropical dry and moist broadleaf forests. VPD: vapour pressure deficit, MCWD: maximum climatic water deficit, CEC: cation exchange capacity, Δ: change.
Extended Data Fig. 3 Spatial distribution of climatic and soil conditions across the tropical and subtropical dry and moist broadleaf forests.
MCWD: maximum climatic water deficit, VPD: vapour pressure deficit, CEC: soil cation exchange capacity, Clay: soil clay content. Δ: change.
Extended Data Fig. 4 Principal component analysis of the distribution of the plot locations in environmental space.
The PCA in (a) shows the distribution of plots in climatic and (b) in soil space. MCWD: average Maximum Climatic Water Deficit and VPD: average Vapour Pressure Deficit, ΔMCWD and ΔVPD: change in MCWD and VPD respectively between the 1958–1987 and 1988–2017 period. MCWD and VPD represent the full-term climatic conditions (1958–2017 period). CEC: cation exchange capacity and soil pH are highly correlated and only CEC is used for further analysis. Clay and sand are highly correlated and only clay is used for further analysis. Coloured ellipsoids in a) and b) encompass 95% of the distribution of the vegetation plots from each field sampling location.
Extended Data Fig. 5 Spatial predictions of functional diversity (FD) depicting the locations of vegetation plots (blue crosses) that were used to fit the statistical models.
The spatial predictions of morphological/structural (top panel), nutrients (middle panel) and photosynthetic (bottom panel) traits are shown. For details about the plots, their location and climatic and soil conditions see Supplementary Table S1).
Extended Data Fig. 6 Spatial predictions of functional redundancy (FRed) depicting the locations of vegetation plots (blue crosses) that were used to fit the statistical models.
The spatial predictions of morphological/structural (top panel), nutrients (middle panel) and photosynthetic (bottom panel) traits are shown. For details about the plots, their location and climatic and soil conditions see Supplementary Table S1).
Extended Data Fig. 7 Bivariate maps combining the functional diversity (FD) and redundancy (FRed) for the morphological traits.
Each map shows the predictions obtained using the full dataset (full model, top panel) and the changes that occur by leaving the plots from each continent out of the model. The second panel shows the spatial predictions when leaving the records from the Americas out of model fitting, the third panel when leaving records from Africa out and the bottom panel when leaving the records from Asia and Australia out from model fitting.
Extended Data Fig. 8 Bivariate maps combining the functional diversity (FD) and redundancy (FRed) for the nutrients traits.
Each map shows the predictions obtained using the full dataset (Full model) and the changes that occur by leaving the plots from each continent out of the model. The second panel shows the spatial predictions when leaving the records from the Americas out of model fitting, the third panel when leaving records from Africa out and the bottom panel when leaving the records from Asia and Australia out from model fitting.
Extended Data Fig. 9 Bivariate maps combining the functional diversity (FD) and redundancy (FRed) for the photosynthesis traits.
Each map shows the predictions obtained using the full dataset (Full model) and the changes that occur by leaving the plots from each continent out of the model. The second panel shows the spatial predictions when leaving the records from the Americas out of model fitting, the third panel when leaving records from Africa out and the bottom panel when leaving the records from Asia and Australia out from model fitting.
Supplementary information
Rights and permissions
About this article
Cite this article
Aguirre‐Gutiérrez, J., Berenguer, E., Oliveras Menor, I. et al. Functional susceptibility of tropical forests to climate change. Nat Ecol Evol 6, 878–889 (2022). https://doi.org/10.1038/s41559-022-01747-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01747-6
This article is cited by
-
Contrasting carbon cycle along tropical forest aridity gradients in West Africa and Amazonia
Nature Communications (2024)