Abstract
Seagrasses are among the most efficient sinks of carbon dioxide on Earth. While carbon sequestration in terrestrial plants is linked to the microorganisms living in their soils, the interactions of seagrasses with their rhizospheres are poorly understood. Here, we show that the seagrass, Posidonia oceanica excretes sugars, mainly sucrose, into its rhizosphere. These sugars accumulate to µM concentrations—nearly 80 times higher than previously observed in marine environments. This finding is unexpected as sugars are readily consumed by microorganisms. Our experiments indicated that under low oxygen conditions, phenolic compounds from P. oceanica inhibited microbial consumption of sucrose. Analyses of the rhizosphere community revealed that many microbes had the genes for degrading sucrose but these were only expressed by a few taxa that also expressed genes for degrading phenolics. Given that we observed high sucrose concentrations underneath three other species of marine plants, we predict that the presence of plant-produced phenolics under low oxygen conditions allows the accumulation of labile molecules across aquatic rhizospheres.
Similar content being viewed by others
Main
Seagrasses are marine angiosperms that form the foundation of ecologically and economically valuable ecosystems along coastal regions of all continents except Antarctica1. As ecosystem engineers, seagrasses provide important services to humans. For example, seagrass meadows are important habitats for fisheries, stabilize the sea floor, remove pollutants and drive biogeochemical cycling2. Seagrasses are highly efficient in sequestering carbon from the atmosphere by incorporating it into their tissues and burying it as organic matter in their sediments3; they bury carbon 35 times faster than rainforests per unit area4. Seagrasses also excrete high concentrations of dissolved organic carbon (DOC) into the environment5, which is assumed to be partially metabolized by the microorganisms in the sediments surrounding seagrass rhizomes and roots.
On land, angiosperms excrete up to 30% of their primary production as organic compounds to their soils to attract beneficial microbial partners, to defend themselves from pathogens and to communicate with other individuals6. These root exudates feed complex microbial food webs that drive the long-term storage of organic carbon7. Much less is known about how seagrasses interact with the microbial communities in their sediments8. Here, we show that seagrass sediments are veritable sweet spots in the sea that contain surprisingly high concentrations of simple sugars, primarily in the form of sucrose. Our results indicate that microbial consumption of these reactive sources of organic carbon is inhibited by the presence of phenolic compounds in the seagrass rhizosphere.
Results and discussion
Posidonia oceanica sediments are rich in sugars
Our metabolomic analyses of pore water chemistry underneath a meadow of the endemic Mediterranean seagrass, P. oceanica, unexpectedly revealed concentrations of sugars in the high µM range (Fig. 1a,b, Supplementary Fig. 1 and Supplementary Tables 1 and 2). The primary sugar in the pore water metabolome was the disaccharide sucrose (Supplementary Fig. 2), which also has been shown to be the most abundant sugar in the tissues of seagrasses, including P. oceanica9. Given that seagrasses occupy 300,000–600,000 km2 of coastal regions3, we conservatively estimated a global stock of 0.67–1.34 Tg of sucrose in the upper 30 cm of seagrass sediments. The sucrose concentrations we measured underneath P. oceanica are as much as 80 times higher than previously measured dissolved total carbohydrate pools from marine sediments and 2,000-fold higher than free sugar concentrations in the open ocean10,11. Our observations represent a paradox in microbial ecology, as it is widely known that most microorganisms quickly consume sugars, like sucrose, in their immediate environment12,13. Until now, the only known microbial habitats that contain similarly high concentrations of simple sugars include saps and fruit nectars, rhizospheres of agro-ecosystems including sugar beets and biofilms on mosses and marine macroalgae14,15.
a, Sediment pore water profiles were collected 20 m away, 1 m away and underneath a Mediterranean P. oceanica meadow off Elba, Italy. Pore water profiles consisted of samples collected every 5 cm from 0 to 30 cm sediment depth. b, Plots show sucrose concentrations in pore waters across sediment depth (replication numbers are reported for each location and sample depth combination). Red points represent mean concentrations ± s.e.m. Sample sizes at each sediment depth are indicated in the figure. Sucrose concentrations are plotted on a log scale. Sugar concentrations were significantly higher inside the meadow than outside or at the edge (P = 3.56 × 10−13; two-way ANOVA comparing sugar concentrations as a function of sediment depth and location; model results and significance values are reported in Supplementary Tables 2 and 3, average values across sediment depths are reported in Supplementary Table 1). c–e, Schematic, light microscopy (c,d) and matrix-assisted laser desorption/ionization MS images (e) from a P. oceanica root collected from a meadow in the Mediterranean off Elba, Italy. Displayed is a quarter of a root cross-section. In e, the molecular ion distribution of disaccharides (C12H22O11, potassium adduct [M + K]+, m/z 381.0754), including sucrose (as confirmed by GC–MS analyses), shows that the relative abundance was highest in the root rhizodermis. Replicate measurements (n = 7) of individual root sections are presented in Supplementary Fig. 5. Relative abundances are visualized as a heatmap from low (blue) to high (yellow) intensities of each pixel (10 µm). rd, rhizodermis; hd, hypodermis; c, cortex; l, lacunae; ed, endodermis; s, stele.
To understand sugar distribution across spatial scales, we collected 570 metabolomic profiles from sediment pore water underneath, 1 m and 20 m away from a P. oceanica meadow in the Mediterranean (in, edge and out; Fig. 1a). Non-targeted and targeted analyses revealed that sediments underneath P. oceanica contained significantly higher concentrations of sucrose, glucose, trehalose, myo-inositol and mannitol compared to the sites away from the seagrass (Extended Data Fig. 1, Supplementary Fig. 1 and Supplementary Tables 2 and 3). Bulk measurements of DOC were at least 2.6× higher inside the meadow than at the edge or outside (Extended Data Fig. 2a and Supplementary Tables 2–4). Measured sugars contributed up to 40% of the DOC pool underneath seagrass meadows, which form an energy-rich source of carbon that has not yet been described or characterized (Extended Data Fig. 2b)5. Previous studies showed that seagrasses excrete organic carbon produced from CO2 fixation into their sediments16 and hypothesized that sucrose is one of the major components of the excreted carbon given its high concentrations in seagrass tissues9. However, until now the molecular composition of the organic carbon excreted by seagrasses was not known. Here, we provide evidence that sucrose is the major component of P. oceanica exudates and, in the following, link seagrass primary production to the accumulation of sucrose in the marine rhizosphere.
Through a series of temporal and spatial analyses, we provide evidence to support our assumption that sucrose enters the rhizosphere through P. oceanica’s roots. First, field experiments showed that sucrose concentrations differed across seasonal and daily time scales (Supplementary Text 1, Supplementary Fig. 3 and Extended Data Fig. 3) and were highest at the sediment depths where the seagrass roots occurred (Fig. 2a–c and Supplementary Fig. 4). Second, mass spectrometry (MS) images of cross-sections of P. oceanica roots revealed that sucrose was distributed throughout the root tissues. Sucrose was particularly abundant in the rhizodermis, the outermost cell layer of the root (Fig. 1c–e and Supplementary Fig. 5), suggesting that the sugar is excreted or passively released to the sediment through the rhizodermis. Both active transport and diffusion are known from terrestrial plants which release exudates, including sugars, through their roots into soils6,17. Collectively, our results from P. oceanica indicate that this marine plant transfers substantial amounts of sucrose to the surrounding sediments through its roots on a daily basis.
a, Schematic showing that P. oceanica roots extend between 5 and 20 cm into the sediments in Mediterranean seagrass meadows off the Island of Elba, Italy. b,c, DOC (Supplementary Table 4) (b) and sucrose concentrations (c) were highest in the zone of root penetration between 5 and 30 cm (two-way ANOVA P < 0.001; ANOVA model results and post-hoc tests reported in Supplementary Tables 2 and 3). Red points represent mean ± s.e.m. DOC and sucrose concentrations across sampling depth; red lines connect the means. Replication numbers across sediment depths are indicated next to b and c. d, Oxygen profiles (n = 2) using a lancet to measure O2 in pore waters revealed that oxygen concentrations were highest at the sediment surface, decreased sharply between the upper sediment layers and 2.5 cm and became anoxic at 7.5 cm. Points represent sediment horizon depths where samples were collected; different line types and colours represent individual profiles.
Sucrose respiration is limited in meadow sediments
Sucrose and other sugars are important carbon and energy sources for soil and sediment microorganisms. To understand why seagrass-produced sugars are not quickly consumed by sediment microorganisms, we measured in situ oxygen concentrations in sediments underneath a Mediterranean P. oceanica meadow. We observed a steep decrease in oxygen concentrations, from 202 µM to <0.5 µM, within the first 7.5 cm below the sediment surface reaching hypoxic conditions after 5 cm (7–10 µM, Fig. 2d). In our measurements, oxygen penetrated deeper than the mm depths commonly observed in sediments underneath and adjacent to P. oceanica meadows, although oxygen penetration depths down to 4 cm have been observed18,19,20. Past studies showed that seagrasses, including P. oceanica, release oxygen from their roots to their sediments21 but we did not detect oxygen in deeper sediment layers, presumably because our oxygen sampling protocol could not detect variations in oxygen concentrations at scales <2.5 cm (Supplementary Methods).
The observed decrease in oxygen with depth underneath P. oceanica meadows suggests that microorganisms living in sediment layers deeper than 5 cm are not able to use aerobic pathways for sucrose respiration. In the absence of oxygen, microorganisms ferment sugars to organic acids, which a syntrophic community of bacteria and archaea respire to CO2, mainly using nitrate and sulfate as electron acceptors. While nitrate is limited in sediments underneath seagrass meadows22, sulfate concentrations in Mediterranean pore waters exceed 28 mM and are stable across sites and sediment depths20. It is therefore likely that there was sufficient sulfate underneath P. oceanica meadows to allow sulfate-reducing microorganisms to metabolize the waste products of sugar fermentation. Coupled with previous observations of little to no sulfide underneath these meadows despite the availability of sulfate20, we hypothesize that other biogeochemical factors beyond electron acceptors inhibit the microbial degradation of simple sugars underneath seagrass meadows.
Phenolics are one class of compounds that could limit the microbial degradation of sucrose under anoxic conditions, as shown for ruminal microbiomes where the presence of phenolics inhibits microbial growth23. To test our hypothesis, we analysed the molecular composition of sediment pore water dissolved organic matter (DOM) underneath and adjacent to a Mediterranean P. oceanica meadow using ultrahigh-resolution MS24. We found that, across sampling locations, 10–16% of all detected molecular formulae consisted of polyphenols and other highly aromatic compounds (molecular identification according to ref. 25) (Fig. 3a). The composition of these DOM profiles was comparable to ecosystems with strong inputs of terrestrial organic matter, such as rivers26,27. P. oceanica contains phenolic compounds in its root tissues, including chicoric acid and caffeic acid (Extended Data Fig. 4 and Supplementary Fig. 6)28. Indeed, the sum formula for caffeic acid (C9H8O4) had highest counts in the pore waters underneath the seagrass meadow between 5 and 25 cm sediment depth (Fig. 2b and Supplementary Table 2; see Supplementary Fig. 7 for identification of caffeic acid in sediment pore waters). Likely sources for caffeic acid in the pore waters include the active release from living seagrass or leachates from decaying P. oceanica material. Given that P. oceanica peat persists in sediments for hundreds to thousands of years and that decaying plant material is a source of lignin and phenolic compounds to soils on land, it is likely that decaying seagrass material is one of the major sources of phenolics in sediments underneath and next to the seagrass meadow29,30.
a, Pore water profiles of the molecular DOM composition underneath (in) and 1 m (edge) and 20 m (out) from seagrass meadows, measured by ultrahigh-resolution MS. Our analyses revealed abundant aromatic molecular formulae, including polyphenols, which comprised 10–16% of all molecular formulae across sampling sites. Size of points reflects number of molecular formulae per sample. b, Ion counts of the molecular formula C9H8O4, which includes the phenolic compound most abundant in seagrass tissues caffeic acid, are significantly higher inside the meadow than at the edge or outside the meadow (two-way ANOVA, location P = 0.00026). Count distributions also reveal higher counts within the root–sediment interface, between 5 and 25 cm below the sediment surface (two-way ANOVA, location × depth P = 0.03). Identification of caffeic acid (C9H8O4) in pore waters beneath P. oceanica was confirmed using LC–MS/MS (Supplementary Fig. 7). For statistical results and post-hoc tests see Supplementary Tables 2 and 3.
To test if the presence of seagrass phenolics restricts microbial degradation of sucrose, we extracted phenolics from P. oceanica tissue (Supplementary Fig. 6) and co-introduced this extract with 13C12-sucrose to meadow sediments under oxic and anoxic conditions using slurry experiments (Supplementary Text 2). We observed that the presence of seagrass-derived phenolics under anoxic conditions significantly decreased 13C-sucrose respiration rates and consequently the production of 13CO2 over tenfold. In contrast, under oxic conditions we saw no significant effect of phenolics on sucrose respiration (Fig. 4a,b and Supplementary Text 2). We used the measured respiration rates from our anoxic sediment incubations to compare how quickly the calculated standing stock of sucrose underneath a P. oceanica meadow of 3.99 mmol m−2 in the anoxic sediment layers (5–20 cm) could be completely respired to CO2 in the absence and presence of seagrass-derived phenolics (for calculations see Supplementary Table 5 and Supplementary Text 3). In the absence of phenolic compounds under anoxia, the standing sucrose pool underneath the meadow would be completely respired to CO2 at a rate of 232 mmol(C) m−2 d−1 in seawater (Fig. 4c), resulting in the complete removal of sucrose to CO2 in less than one day (Supplementary Table 5). When we consider the presence of seagrass-derived phenolics, our incubation results showed that under anoxia, phenolics decreased respiration rates of C from 13C-sucrose to 20 mmol(C) m−2 d−1 (Fig. 4c and Supplementary Table 5). Our results are consistent with previous studies showing that phenolic inhibition of microbial respiration increases with decreasing oxygen concentrations31,32,33. They are also consistent with the requirement for oxygen by microbial phenol oxidases (PO), the enzymes central to the microbial oxidation of phenolic compounds. Studies exploring PO activity in peatlands showed that phenolic degradation decreases with decreasing oxygen availability31,34. In P. oceanica meadows, the degradation of phenolics in the oxic layer under the meadow would allow the sediment microbial community to fully respire sucrose, while under low to no oxygen conditions the high concentrations of phenolics inhibit microbial consumption of sucrose and sugars accumulate under the seagrass meadows.
a,b, Sediments from replicate cores (n = 3) collected from inside a Mediterranean P. oceanica meadow off the Island of Elba, Italy, consumed 13C12-sucrose (a) and produced 13CO2 (b) over the course of oxic and anoxic experiments conducted over 24 h. Individual points in both a and b represent treatment means ± s.e.m. across independent replicates (n = 3). Under oxic conditions, the production rate of 13CO2 from 13C12-sucrose was similar across treatments and at least 2.5× higher than rates observed under anoxia (note different y axis scales). However, in the anoxic incubations, the addition of phenolics extracted from P. oceanica tissues inhibited the microbial degradation of sucrose to CO2. c, The estimated measured respiration rates under anoxia show that the production of 13CO2 from sucrose in the presence of phenolic compounds is at least eight times lower than that of natural or artificial seawater condition (Supplementary Table 5). The size of the arrows is proportional to the potential rate of CO2 released from sucrose in mmol(C) m−2 d−1.
Our incubation experiments show that the microbial community underneath seagrass meadows have the metabolic ability to degrade sucrose but this potential is significantly reduced by the presence of phenolics in their environment. Our work, together with research on plant exudation in terrestrial rhizospheres, indicates that both marine and land plants excrete sugars and phenolic compounds to their soils30,35. However, in terrestrial rhizospheres where there is sufficient oxygen, these phenolic compounds are degraded36. This removes the inhibitory effect of most phenolic compounds on microbial metabolism, so that microbial communities are able to consume plant-produced sugars37. In contrast, in the marine rhizosphere of P. oceanica, only the first few centimetres underneath the meadow are oxic, while most of the sediment underneath the meadows is anoxic. In these anoxic sediments, seagrass-produced phenolics are not quickly oxidized and thus accumulate, limiting the microbial degradation of sucrose and allowing sugars to accumulate in the seagrass rhizosphere. Thus, the presence of phenolics and the lack of oxygen underneath seagrasses delays the input of reactive, organic carbon into microbial respiration, allowing sucrose to accumulate.
Most seagrass rhizosphere microorganisms do not use sucrose
Plant carbon deposition to terrestrial soils serves as a chemo-attractant for beneficial microorganisms, including arbuscular mycorrhizas and beneficial bacteria and archaea6. We hypothesize that seagrass deposition of sucrose, phenolics and other compounds into sediments, structures the composition and function of the associated marine rhizosphere.
Using metagenomics and full-length 16S rRNA amplicon sequencing from sediments collected inside and 1 m and 20 m outside the meadow, we show that the sediments underneath seagrass meadows contained a distinct community of microorganisms (Fig. 5a,b and Extended Data Fig. 5). Using principal coordinate analyses (PCoA), samples clustered according to their collection site, on the basis of the taxonomic composition of both metagenomic and full-length 16S rRNA sequencing libraries (Fig. 5a,b). Our amplicon sequencing further revealed that bacterial communities were highly diverse across collection sites, mirroring known complexities in terrestrial habitats that contain thousands of taxa per gram of soil38. However, we observed fewer amplicon sequence variants (ASVs) inside than outside of the meadow (Fig. 5c). These data indicate that the presence of the seagrass rhizosphere not only selects for specific taxa but also limits the diversity of the community. Similarly, on land, plants secrete compounds to their rhizospheres, creating a habitat that shapes the community composition of their soil taxa39 and results in a specialized microbial community with lower diversity than neighbouring unvegetated soils40,41.
a,b, PCoA analyses show that the taxonomic composition of microbial communities underneath (in) and away (edge and out) from a Mediterranean P. oceanica meadow off Elba, Italy, were significantly different on the basis of bulk metagenomics (order level, one-way analysis of similarities (ANOSIM) R2 = 0.65, P = 0.006) (a) and full-length 16S rRNA amplicon sequencing (genus level, one-way ANOSIM R = 0.835, P = 0.005) (b). c, The total number of ASVs was significantly lower in samples collected inside and outside the meadow than at the edge (Min = 1,793 ± 79 s.e.m.; Medge = 2,224 ± 23 s.e.m.; Mout = 1,983 ± 51 s.e.m.). NS, not significant. d, Boxplots show the accumulative expression ratio in each MAG between glycoside hydrolases (GH) predicted to degrade sucrose compared to other sugars (overlying points). Putative sucrose specialists (coloured points) are defined as having higher TPMs for sucrose degradation compared to all other GH enzymes (TPMsucrose/TPMother > 1). TPMs represent the total expression across each site-specific library. The majority of MAGs across sites were sugar generalists, while putative sucrose specialists were only found in three MAGs inside and three at the edge of the meadow. e–j, Phylogenomic trees for six sucrose specialists: MAG 142 (e); MAG 209 (f); MAG 438 (g); MAG 154 (h); MAG 207 (i); and MAG 76 (j). Coloured boxes show members that grouped within the same family clade. For the relative abundance of each of the putative sucrose specialists across habitats see Supplementary Fig. 10.
Microbial genomes recovered from our metagenomic analyses (Supplementary Fig. 8) revealed that taxa in the P. oceanica rhizosphere appear to be limited in their ability to degrade sucrose in spite of the high abundance of this simple sugar in their environment. We recovered 182 metagenomic assembled genomes (MAGs), 125 of which were of medium draft quality (Supplementary Tables 6 and 7) and focused our metagenomic and metatranscriptomic analyses on genes used in microbial sucrose metabolism. Although 80% of the recovered MAGs contained genes that encode for sucrose degradation, including sucrose phosphorylases and sucrose-6-phosphate hydrolases, these genes were expressed in only 64% of the MAGs (Supplementary Table 8). Furthermore, for most MAGs, the expression of genes that encoded for sucrose degradation were not among the top 10% of all expressed orthologues (Supplementary Text 4 and Supplementary Fig. 9).
Despite the relatively low expression of genes that encode for sucrose degradation across most MAGs, we identified six MAGs across sampling sites that expressed a higher proportion of genes encoding for sucrose degradation compared to other sugars (TPMsucrose/TPMother > 1; Fig. 5d and Extended Data Fig. 6). On the basis of our genomic analyses, we predict that three of these six bacterial species preferentially metabolize sucrose over other sugars inside the seagrass meadow. These putative sucrose specialists were undescribed members of the Xanthomonadales (MAG 142), Verrucomicrobiales (MAG 209) and environmental gammaproteobacterial lineage UBA6522 (MAG 438) (Fig. 5e–g). The putative sucrose specialists at the edge of the meadow belonged to the Desulfobacterales (MAG 154), Thiohalomonadales (MAG 207) and Beggiatoales (MAG 76) (Fig. 5h–j). In addition to sucrose degradation, these six MAGs covered a range of metabolic pathways, including fixation of CO2 and N2, the use of reduced sulfur compounds and hydrogen as energy sources and sulfate respiration (Supplementary Table 8 and Supplementary Text 4). Both MAGs 76 and 154 are facultative anaerobes on the basis of the absence of cytochrome c oxidase in their genomes.
Given our experimental results showing that phenolic compounds inhibited microbial sucrose degradation under anaerobic conditions, we assume that anaerobic sucrose degraders should be able to degrade phenolics. Indeed, expression data indicated that both MAG 76 and MAG 154 degrade phenolics through the benzoyl-CoA and beta-ketoadipate pathways (Supplementary Table 8). Furthermore, expression data for three of the four aerobic MAGs indicated that these also degrade phenolics, allowing them to metabolize sucrose in the upper layers of the seagrass rhizosphere (Supplementary Text 4). It is intriguing that the N2-fixing symbiont in the root tissues of P. oceanica, which also gains its energy and carbon from plant-derived sugars, expresses genes involved in detoxifying phenolic compounds19, indicating the strong selective advantage this trait provides for seagrass-associated microorganisms.
Our metagenomic and metatranscriptomic results are consistent with our sediment incubation experiments. Many members of the microbial community underneath the meadow have the genomic ability to degrade sucrose. However, low transcription of genes involved in sucrose metabolism, together with our measurements of high sucrose concentrations underneath the meadow, indicate most members of the seagrass rhizosphere do not actively degrade this carbon source. We hypothesize that the few taxa that disproportionately expressed genes for sucrose degradation over other sugars evolved mechanisms that enabled them to thrive on this abundantly available and easily degradable energy and carbon resource in the seagrass rhizosphere despite the presence of phenolic compounds. This is consistent with the wealth of data from terrestrial rhizospheres showing that select microorganisms are able to use sugars and other compounds in the presence of phenolics36,37,42. In fact, in terrestrial rhizospheres, phenolic compounds work to shape soil community composition by acting as antimicrobials towards some microbial taxa while stimulating others42,43.
Sucrose persists in other aquatic rhizospheres
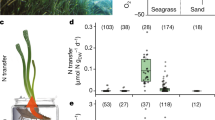
We hypothesize that other types of aquatic rhizosphere also contain high concentrations of sucrose and other sugars, given that most plants produce phenolic compounds and oxygen is rapidly depleted in the first few centimetres in aquatic sediments44,45. We measured similarly high concentrations of sucrose as those in P. oceanica meadows in the pore waters of other marine plant ecosystems: in two other seagrass species from phylogenetically distinct lineages of angiosperms (Thalassia testudinum and Syringodium filiforme) and in a mixed stand of mangrove species (primarily Rhizophora mangle) (Fig. 6 and Supplementary Table 1). It is intriguing that sucrose persists in these otherwise oligotrophic ecosystems. To fully understand how these labile components of the DOC pool integrate into global carbon models, it is critical that future studies quantify the composition of this otherwise unrecognized carbon pool.
a, Seagrasses occur in coastal waters worldwide (data accessed from UN Environmental World Conservation Monitoring Center on 21 March 2020, http://data.unep-wcmc.org/datasets/7). S. filiforme (pink), T. testudinum (yellow) in Belize (BZ) and P. oceanica (purple) in Italy (IT) were the dominant species at both sampling sites in this study. b, Box-and-whisker plots with overlaying data points show that pore waters collected underneath seagrass meadows and mangroves varied, depending on the type of meadow. Centre line is the median, box limits represent the upper and lower quartiles, whiskers are 1.5× the interquartile ranges, outliers are not shown. Specifically, sucrose was significantly higher in pore waters underneath the mixed (n = 14) and mono stands of the seagrass species S. filiforme (n = 29) and T. testudinum (n = 47), followed by samples taken underneath the mangrove peat (n = 48) and finally were lowest underneath P. oceanica meadows (n = 382). In almost all cases, excluding the samples from the mangroves, sucrose concentrations sometimes exceeded micromolar values. Post-hoc tests results: ***P < 0.001, **P < 0.01. Contrasts not shown are not significant (Supplementary Tables 2 and 3).
Conclusions
Our study revealed that large amounts of the sucrose produced by P. oceanica did not fully enter the microbial loop, which probably has significant consequences for carbon burial in these coastal ecosystems. The accumulation of sucrose is driven by the presence of phenolics under hypoxic to anoxic conditions, which we show limited microbial metabolism of sugars. These results provide evidence that the reactivity of DOM is complex and governed by biotic and abiotic processes, including the metabolic potential of the microbial community, the availability of electron acceptors and inhibitors of microbial metabolism.
As a valuable energy resource, why does P. oceanica deposit sucrose to their sediments? P. oceanica release of sugars is probably an evolutionary mechanism to deal with overflow metabolism during periods of high productivity. Seagrasses evolved a reduced capacity to produce structural carbohydrates, such as cellulose, in favour of maintaining a large osmolyte pool of non-structural carbohydrates, such as sugars, to compensate for high salinity compared to their terrestrial relatives46. Seagrass release of excess photosynthate to their sediments in the form of sucrose could also serve as an overflow valve when productive rates exceed storage capacity or when nitrogen and phosphorus limit growth. In most marine oxygen-limited environments, plant release of sugars would stimulate sulfate reduction by sediment microbes leading to the accumulation of high concentrations of sulfide, which is toxic to seagrass roots47,48,49. However, we show that the presence of phenolics limits the microbial respiration of sugars under these low oxygen conditions. This would have the selective advantage that seagrasses could deposit their excess photosynthates to their sediments, while limiting the formation of sulfide.
Another selective advantage for P. oceanica in releasing sucrose is that it probably attracts key microbial partners that benefit plant fitness within the seagrass rhizosphere. In terrestrial plants, sucrose makes up 50% of their exudates during their early developmental stages. The early priming of the plant’s rhizosphere with sugar is critical in shaping plant-associated microbial communities35. Plant exudation of sugars and other compounds is tied to chemotaxis of potential symbionts, including soil bacteria and mycorrhizal fungi42. Here, we show that in the seagrass rhizosphere, the vast majority of sediment microorganisms were not able to metabolize sucrose. However, we did identify a select set of putative sucrose specialists that have the metabolic potential to degrade phenolics and consume sucrose. Some of these taxa are also capable of sulfur and hydrogen oxidation and, intriguingly, nitrogen fixation. In oligotrophic waters, such as the Mediterranean, nitrogen is a limited resource for growth. Sucrose release by seagrasses, including P. oceanica, to their rhizosphere could support beneficial symbionts that metabolize sucrose and fix nitrogen in the presence of phenolics and absence of oxygen.
Future works needs to define the composition and decomposition rates of the DOC pool underneath aquatic plants and determine the lability of DOC as well as the proportion of buried carbon in seagrass and mangrove environments. This includes quantifying the rates at which aquatic plants excrete sucrose and other sugars to their rhizosphere using stable isotope probing to track the production and subsequent release of labelled metabolites16,50. Given that many plants excrete sugars and phenolics to their soils, and that in most aquatic environments sediments are largely anoxic, we predict that future studies will discover high concentrations of sugars in other rhizosphere environments. In addition to seagrasses and mangroves, this will include the rhizosphere of rice, marshlands and swamps. This study lays the foundation for future research to explore the processes that promote the burial and persistence of normally labile carbon and expand our understanding of carbon cycling in aquatic rhizospheres.
Methods
Pore water sampling
Pore water was collected from vegetated and non-vegetated sediments for metabolomic analyses from P. oceanica seagrass meadows in the Mediterranean off the island of Elba, Italy (42° 48′ 29.4588″ N; 10° 8′ 34.4436″ E), the Caribbean at Carrie Bow Cay, Belize (N 16° 04′ 59″; W 88° 04′ 55″) and Twin Cayes, Belize (N 16° 50′ 3″; W 88° 6′ 23″) and the Baltic Sea off the coast of Kiel, Germany (54° 27′ 26.56256″ N; 10° 11′ 33.1908″ E). All water samples were collected using a blunt steel lance. For details regarding the experimental design for both spatial and temporal sampling of pore water and seagrass tissues see Supplementary Methods.
Gas chromatography–mass spectrometry-based metabolomics
Metabolomic profiles were obtained from sediment pore waters and prepared for gas chromatography–mass spectrometry (GC–MS) using a recently described method51. This method targets the quantification and identification of lower weight molecular compounds. It is possible that there are other higher molecular weight metabolites, such as oligosaccharides and polysaccharides, dissolved in the pore water that were not captured by our analytical approach in the pore water samples. Briefly, 0.5 ml of individual pore water samples were dried to completeness in a speed-vacuum concentrator (~8 h). To further remove water from the salt pellet, we added 250 µl of toluene to the dried pellet and sonicated the mixture for 10 min. The toluene was subsequently removed under a steady flow of N2 gas. The resulting extracts were stored until GC–MS analysis at 4 °C.
Sample derivatization
To remove condensation formed during extract storage, directly before preparation for GC–MS analysis we further dried extracts in a vacuum concentrator for 30 min. Metabolite derivatization was performed by adding 80 µl of methoxyamine hydrochloride dissolved in pyridine (20 mg ml−1) to the dried pellet and incubating for 90 min at 37 °C using a thermal rotating incubator under constant rotation at 1,350 r.p.m. Following recent advancements in signal improvement52, the pyridine was removed from the sample at room temperature under a gentle flow of N2 gas (~1 h). Following the addition of 100 µl of N,O-bis(trimethylsilyl)trifluoroacetamide, each extract was vortexed and incubated for another 30 min at 37 °C using a thermal rotating incubator under constant rotation at 1,350 r.p.m. A total of 100 µl was transferred to a GC–MS vial for further GC–MS data acquisition.
GC–MS data acquisition
To obtain metabolite profiles, all derivatized samples were analysed on an Agilent 7890B GC coupled to an Agilent 5977 A single quadrupole mass selective detector. The injector temperature was set at 290 °C. Using an Agilent 7693 autosampler, 1 µl was injected in splitless mode through a GC inlet liner (ultra inert, splitless, single taper, glass wool, Agilent) onto a DB-5MS column (30 m × 0.25 mm, film thickness 0.25 µm; including 10 m DuraGuard column, Agilent). Metabolite separation on the column was achieved with an initial oven temperature of 60 °C followed by a ramp of 20 °C min−1 until 325 °C was reached, which was then held for 2 min. Helium carrier gas was used at a constant flow rate of 1 ml min−1. Mass spectra were acquired in electron ionization mode at 70 eV across the mass range of 50–600 m/z and a scan rate of two scans per second. The retention time was locked using standard mixture of fatty acid methyl esters (Sigma-Aldrich).
Metabolome data analysis
To identify differences in metabolites between vegetated and non-vegetated sediments, we used a non-targeted metabolomic approach to assess pore water samples collected inside and outside a P. oceanica seagrass meadow. Raw GC–MS data from samples collected in October 2016 were imported into R (v.3.5.2) as converted mzXML files (MsConvert)53 and processed using XCMS (v.2.99.6)54. For further details on GC–MS data processing, see Supplementary Methods.
Following data processing, the resulting ion abundances from individual peaks were normalized to the ribitol internal standard and compared using a volcano plot to show ions with significant differences (alpha < 0.1) between sampling location and high fold change values (log(FC) > 2). These peaks were subsequently identified using the Mass Hunter Suite through comparison to the NIST database. The metabolite ID was confirmed by comparing peak retention times and mass spectra to authentic standards run on the same instrument.
Using our non-targeted analysis as a guide, identified sugars were further quantified across metabolomic profiles using Mass Hunter Quantification Suite (Agilent). Salinity matched calibration curves were used to determine absolute sugar concentrations in pore water samples using the same derivative across samples for each compound. Values below the detection limits were considered noise and given a value of 0 µM. The relative ion abundances of plant sugars were normalized to tissue wet weight and compared using an analysis of variance (ANOVA) across sampling time. Sugar concentrations from sediments were compared across spatial and temporal scales using ANOVA models where sugar concentration was a function of (1) sampling location ×sampling depth, (2) sampling month and (3) time of day blocked by sampling spot. Sugar concentrations were transformed to meet the assumptions of the model.
Estimation of global sucrose abundances
To determine the amount of sucrose underneath seagrass meadows in sediment pore waters, we first calculated the total volume of pore water underneath meadows worldwide for the estimated range in known area of seagrasses (300,000–600,000 km2) (ref. 3). For both the estimated lower and upper surface areas of seagrass, we calculated the total volume of pore water using the following equation:
where we used the value of 0.661 for median porosity of sediments worldwide3 and a sediment depth of 0.0003 km for the measured distribution of sucrose based on our measurement in this study. The calculated minimum and maximum l of pore water were subsequently used to determine the total grams of sucrose using the following equation:
The mean sucrose concentration (35.4 µM) was calculated by taking the average of measured values above the detection limits (>0.02 µM) from sediment pore waters underneath all seagrasses in this study.
DOC sampling and analysis
DOC samples were collected in parallel with pore water metabolomic samples from inside, at the edge and 20 m outside a P. oceanica seagrass meadow in October of 2016. Taking care to avoid oversampling pore water space, ~20 ml of each pore water sample was collected into polypropylene syringes. Samples were filtered through precombusted (500 °C, 4 h) Whatman GF/F filters (0.7 µm) into 20 ml acid-washed and precombusted scintillation vials. Samples were acidified to pH 2 using 25% hydrochloric acid and stored at 4 °C until analysis. DOC concentrations were measured by high-temperature catalytic oxidation on a Shimadzu TOC-VCPH analyser. Instrument trueness and precision were tested against Deep Atlantic seawater reference material (42 µM DOC; acquired from the Hansell laboratory at University of Miami, FL, USA) and low carbon water (1 µM DOC) and was better than 5%.
Molecular DOM analysis
DOM samples were collected in parallel with pore water DOC samples from inside, at the edge and 20 m outside a P. oceanica seagrass meadow in October of 2016. DOM was extracted from filtered (0.7 µm GF/F) and acidified (pH 2) pore water samples using solid-phase extraction (SPE)24. For additional details regarding DOM analysis see Supplementary Methods.
Seagrass root MS imaging
Seagrass root pieces were embedded into precooled (4 °C) 2% carboxymethylcellulose blocks and snap frozen in liquid nitrogen. Roots were cross-sectioned into 12 µm thick slices with a cryrostat (Leica CM3050 S, Leica Biosystems Nussloch; chamber and object holder temperature at −25 °C) and either super 2,5-dihydroxybenzoic acid solution (S-DBH; 30 mg ml−1 in 50% methanol:water with 0.1% trifluoroacetic acid (TFA)) or 9-aminoacridine matrix (9AA) in 70% methanol:water with 0.1% TFA was applied to individual sections using an automated pneumatic matrix sprayer (SunCollect, SunChrome, Wissenschaftliche Geräte). For additional sample preparation details see Supplementary Methods.
MS imaging was performed with an atmospheric pressure matrix-assisted laser desorption/ionization (AP-MALDI) ion source (AP-SMALDI10, TransMIT), coupled to a Fourier transform orbital trapping mass spectrometer (Q Exactive HF, Thermo Fisher Scientific). MS images were collected by scanning the matrix-covered tissue sections at a step size of 10 µm. Mass spectra were acquired in positive-ion mode for all S-DBH-prepared sections with a detection range of m/z 50–750 and a mass resolving power of 240,000 at m/z 200. Sections prepared with 9AA matrix were acquired in negative-ion mode with m/z range 70–1,000 at a 20 µm step size.
Oxygen detection
Oxygen profiling within the seagrass bed was carried out in situ using a combination of custom-built and commercially available instruments on 30 August 2019 (Supplementary Methods gives a description of the instrument and procedure). Scuba divers collected two pore water profiles down to a depth of 30 cm in 2.5 cm intervals.
Microbial activity
Sediments were collected using cores inside (n = 3) and at the edge (n = 3) of a P. oceanica meadow in Sant’Andrea Bay, Elba, Italy on 27 August 2019 (42° 48′ 29.4588″ N; 10° 8′ 34.4436″ E; 6–8 m water depth). Individual cores were subsampled inside a glove bag under nitrogen gas to avoid introducing oxygen to the anoxic sediment layers. Cores were split into oxic (upper 3 cm) and anoxic fractions (5–10 cm). Individual fractions were mixed and 10 ml of sediment volume were added to each serum vial. Replicate 250 ml serum vials for each fraction were filled with filtered seawater (environmental control), filtered artificial seawater (technical control) or filtered artificial seawater with ~35 µM of phenolic extract prepared from seagrass tissue as previously described (Supplementary Methods)55.
We introduced an equal amount of phenolics and sugars as this ratio was reported for other seagrass leachates30. Assuming the main phenolic compound is chicoric acid (Supplementary Fig. 6), this corresponds to a concentration of ~35 µM. All serum vials had 100 ml of headspace with either N2 gas (anoxic) or air (oxic). To remove background levels of phenolics and other compounds, sediments incubated with artificial seawater were flushed with five times the sediment volume in artificial seawater before transfer to serum vials. Flushing with artificial seawater may have inadvertently removed additional cells, nutrients or metals that could have lowered metabolic rates compared to incubations with natural seawater. Fully labelled 13C12-sucrose was added to each bottle at a final concentration of 50 µM. Oxic incubations were prepared in ambient air, with optode spots confirming the presence of oxygen throughout the experiment. Anoxic incubations under nitrogen atmosphere and both natural and artificial seawater were bubbled with N2 gas until <2 µM of oxygen was measured.
Incubation seawater from each serum bottle was collected for GC–MS-based metabolomics and cavity ring-down spectrometry (CRDS). At each time point, 5 ml of incubation seawater were collected from each vial and replaced by either filtered air (oxic) or N2 gas (anoxic). A total of 2 ml was transferred to an Eppendorf tube and stored at −20 °C until analysis (GC–MS), while the remaining 3 ml were transferred to a nitrogen-filled exetainer with 50 µl of saturated mercury(II) chloride solution to halt microbial metabolism. All samples where evidence indicated microbial metabolism was not successfully halted (cross-validated using metabolomics results) were excluded from the CRDS analysis.
The metabolic activity of the sediment was assessed by monitoring the disappearance of 13C12-sucrose (GC–MS) and the production of 13CO2 (CRDS) over time. Samples were prepared for GC–MS following the metabolomics protocol for seawater described above. To determine the rate of 13CO2 production from 13C12-sucrose, 3 ml of each sample were acidified with 50 µl of 20% phosphorphic acid in preparation for cavity ring-down spectroscopy (G2201-i coupled to a Liaison A0301, Picarro, connected to an AutoMate Prep Device, Bushnell)56. The production of 13CO2 from 13C-sucrose was calculated by subtracting the initial 13CO2 concentration from each subsequent time point for each bottle. The production of 13CO2 from 13C12-sucrose was calculated from the increase of 13CO2 over 24 h. The values were corrected by subtracting the 12CO2 background multiplied by the natural abundance of 13C.
Volumetric sucrose oxidation rates were determined by linear regression of the measured 13C12-sucrose and 13CO2 concentrations. The rates from the incubations were corrected for dilution and sediment porosity assumed as 0.42 (on the basis of previous calculations). A correction based on the measured 12C12-sucrose concentration was applied to account for dilution of the labelled substrate. An active layer thickness of 3 cm for oxic and 20 cm for anoxic was assumed on the basis of the average sucrose concentrations in the sediment and multiplied with the volumetric rates (Supplementary Table 5 gives rate calculations).
Detection of phenolic compounds from pore water
We verified the correct identification of the phenolic compound caffeic acid (sum formula C9H8O4) in our ultrahigh-resolution MS analyses of pore water DOM, using liquid chromatography–mass spectrometry (LC–MS) (Supplementary Fig. 7). About 0.5 ml of pore water were collected from underneath a seagrass meadow in July 2016 and processed as described above in ‘Molecular DOM analysis’. After eluting samples from the SPE cartridge with methanol, samples were dried under vacuum for 8 h at 30 °C using the V-AL mode. Resulting extracts were redissolved in 500 μl of methanol, dried down in LC–MS Ultra High Recovery vials, redissolved in 50 μl of 10% methanol:90% MQ H2O and analysed directly by LC–MS (Supplementary Methods).
Sediment nucleic acid extraction, sequencing and analysis
Genomic DNA and total RNA were extracted from sediment cores (n = 3) collected inside, at the edge and 20 m outside a P. oceanica meadow in Sant’Andrea Bay, Elba, Italy, in October 2016. We explored the microbial communities at 10–15 cm because this sediment depth contained the highest sucrose concentrations in pore water profiles and was the average depth of P. oceanica root penetration. Directly after collection, cores were sectioned into 5 cm slices and frozen at −20 °C. A subsample of each sediment slice was also presevered in RNAlater (Sigma-Aldrich) for RNA extraction. Samples were stored at −80 °C until further processing.
Nucleic acid extraction
DNA was extracted from 0.2 g of the 10–15 cm slice. Before extraction, extracellular DNA was removed from the sediments following previously described methods57,58. Total RNA was extracted from a separate 0.2 g aliquot of the 10–15 cm slice of each sediment core preserved in RNAlater following a modified version of previously described methods for RNA recovery from marine sediments59. For additional details of DNA and RNA extraction, see Supplementary Methods.
Library preparation and sequencing
Library preparation and sequencing was performed at the Max Planck Genome Center Cologne, Germany (https://mpgc.mpipz.mpg.de/home/) (Supplementary Methods).
Taxonomic composition using 16S amplicon reads
Resulting 16S CCS reads from our PacBio sequcing approach were processed using Dada2 (ref. 60) in R to obtain ASVs. Briefly, amplicon reads were imported into R and filtered to control for expected sequence length (1,000–1,600 base pairs) and high-quality reads (minQ = 3). Following error estimation, taxonomy was assigned to each unique ASV by comparison against the GTDB database. The resulting count table was imported into phyloSeq61 for subsequent analyses including estimate of sequence richness and comparison of taxonomic profiles across samples.
Taxonomic composition from metagenomic libraries
The pipeline phyloFlash (v.2.0)62 was used to assemble the small subunit of the rRNA gene from each metagenomic library to assess taxonomic composition. The resulting sequence counts, taxonomically classified at order level through comparison to the SILVA SSU 111 (July 2012)63 database, were imported into R (v.3.5.2) and processed using the phyloseq package (v.1.26.1)61 to facilitate analysis and visualization. Data processing included removal of eukaryotic taxa and normalization of count data to relative abundances. A PCoA was used to assess differences in taxonomic compositions among sampling sites.
Metagenomic bin recovery
Metagenomic libraries were used to recover metagenome assembled genomes (MAGs) from marine sediments. Using the bbtools suite (v.38.34, http://jgi.doe.gov/data-and-tools/bb-tools/) reads were cleaned of Illumina adaptors and low-quality ends were trimmed before normalization to a maximum k-mer depth of 100X (k-mer length of 31). Reads with an average k-mer depth <2 were considered erroneous and were discarded. SPAdes (v.3.12.0)64 was used to error-correct all remaining reads, which were then fed into MEGAHIT (v.1.1.3)65 to generate a co-assembly with k-mers from 21 to 51 in steps of 10. Sequencing coverage of each contig was calculated by mapping the error-corrected reads back to the co-assembly using bbmap (v.38.34, http://jgi.doe.gov/data-and-tools/bb-tools/).
The metagenomic co-assembly was binned using three automated binning programs (MetaBAT v.0.32.5, concoct v.1.0.0 and MaxBin v.2.2.6)66,67,68 and subsequently dereplicated and aggregated with DAS Tool (v.1.1.1)69. Bin quality was assessed using the CheckM lineage-based work flow method (v.1.0.7) (Supplementary Table 7)70. Bins with completeness ≥50% and contamination scores corrected for strain heterogeneity ≤10% were considered to be of medium draft quality and used in subsequent analyses. Resulting MAGs were taxonomically classified using the GTDBTk classify work flow (v.0.2.1)71. MAGs represented only 2.9% of the metagenomic read set; however, their taxonomic identities reflected the relative abundance of the sediment communities occurring across habitats (Supplementary Table 7 and Extended Data Fig. 5). Open reading frames from each MAG were predicted with Prodigal (v.2.6.4)72 and annotated using a combination of reference database searchers: dbCAN (v.2.0)73, diamond blastp (v.0.9.25)74, Uniprot75, interproscan (v.5.36-75)76 against both PFam77 and Phobius78 and EggNOG mapper (v.2.0)79.
Expression analysis
Metatranscriptomic reads were cleaned of Illumina adaptors and low-quality ends were trimmed using the bbtools suite (v.38.34, http://jgi.doe.gov/data-and-tools/bb-tools/) before error correction using SPAdes (v.3.12.0)64. Ribosomal RNA was filtered from cleaned reads using SortMeRNA (v.2.1b)80. Non-rRNA reads were mapped to all bins and counted in Kallisto (v.0.46)81. Raw read counts were imported into R (v.3.5.2) for analysis and visualization. To correct for varying relative abundances in MAGs across samples, transcript counts were normalized to one million per each MAG in each sample (transcripts per million, TPM). TPM values for genes with identical annotation were summed within samples. Total transcript counts per habitat per MAG were used to compare transcription levels inside, at the edge and outside the meadow.
Phylogenomic tree construction
Phylogenomic trees were constructed from select MAGs using GToTree (v.1.4.14)82. Briefly, genomes related to each MAG (on the basis of GTDBtk taxonomy) and at least three outgroups were downloaded from NCBI RefSeq or Genbank repositories. Genes were called using Prodigal72 and annotated through HHMER3 (ref. 83) comparisons to a set of 74 bacterial single-copy genes. Genomes were retained in the analysis if they contained at least 45% of all single-copy genes. Annotated genes were aligned using MUSCLE84 and trimmed with TrimAl85. IQ-TREE86 was used to calculate a maximum-likelihood tree with ultrafast bootstrap support values from the concatenated alignment which was visualized using the Interactive Tree of Life webserver87.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Sequence data from this study were deposited in the European Nucleotide Archive under accession numbers PRJEB35096 and PRJEB40297 using the data brokerage service from the German Federation for Biological Data88, in compliance with the Minimal Information about any (X) Sequence (MIxS) standard89. Metabolomics data were deposited in Metabolights (https://www.ebi.ac.uk/metabolights/) under accession numbers MTBLS1570, MTBLS1610, MTBLS1579 and MTBLS1746. All other datasets are available at https://zenodo.org/record/3843378.
Code availability
All code used for data processing and analysis are available at https://github.com/esogin/sweet_spots_in_the_sea.
References
Short, F., Carruthers, T., Dennison, W. & Waycott, M. Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Biol. Ecol. 350, 3–20 (2007).
Barbier, E. et al. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193 (2011).
Fourqurean, J. W. et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 5, 505–509 (2012).
McLeod, E. et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560 (2011).
Barrón, C. & Duarte, C. M. Dissolved organic matter release in a Posidonia oceanica meadow. Marine Ecol. Prog. Ser. 374, 75–84 (2009).
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S. & Vivanco, J. M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266 (2006).
Lange, M. et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 6, 6707 (2015).
Welsh, D. T. Nitrogen fixation in seagrass meadows: regulation, plant–bacteria interactions and significance to primary productivity. Ecol. Lett. 3, 58–71 (2000).
Pirc, H. Seasonal changes in soluble carbohydrates, starch, and energy content in Mediterranean seagrasses. Mar. Ecol. 10, 97–105 (1989).
Burdige, D. J., Skoog, A. & Gardner, K. Dissolved and particulate carbohydrates in contrasting marine sediments. Geochim. Cosmochim. Acta 64, 1029–1041 (2000).
Kirchman, D. L. et al. Glucose fluxes and concentrations of dissolved combined neutral sugars (polysaccharides) in the Ross Sea and Polar Front Zone, Antarctica. Deep Sea Res. II Top. Stud. Oceanogr. 48, 4179–4197 (2001).
Wright, R. R. & Hobbie, J. E. Use of glucose and acetate by bacteria and algae in aquatic ecosystems. Ecology 47, 447–464 (1966).
Decker, E. M., Klein, C., Schwindt, D. & von Ohle, C. Metabolic activity of Streptococcus mutans biofilms and gene expression during exposure to xylitol and sucrose. Int. J. Oral Sci. 6, 195–204 (2014).
Tian, L., Dell, E. & Shi, W. Chemical composition of dissolved organic matter in agroecosystems: correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl. Soil Ecol. 46, 426–435 (2010).
Lievens, B. et al. Microbiology of sugar-rich environments: diversity, ecology and system constraints. Environ. Microbiol. 17, 278–298 (2015).
Moriarty, D. J. W., Iverson, R. L. & Pollard, P. C. Exudation of organic carbon by the seagrass Halodule wrightii Aschers. and its effect on bacterial growth in the sediment. J. Exp. Mar. Biol. Ecol. 96, 115–126 (1986).
Hennion, N. et al. Sugars en route to the roots: transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere. Physiol. Plant. 165, 44–57 (2019).
Weber, M. et al. In situ applications of a new diver-operated motorized microsensor profiler. Environ. Sci. Technol. 41, 6210–6215 (2007).
Mohr, W. et al. Terrestrial-type nitrogen-fixing symbiosis between seagrass and a marine bacterium. Nature 600, 105–109 (2021).
Holmer, M., Duarte, C. M. & Marbá, N. Sulfur cycling and seagrass (Posidonia oceanica) status in carbonate sediments. Biogeochemistry 66, 223–239 (2003).
Pedersen, O., Binzer, T. & Borum, J. Sulphide intrusion in eelgrass (Zostera marina L.). Plant Cell Environ. 27, 595–602 (2004).
Larkum, A. W., Orth, R. J. & Duarte, C. M. Seagrasses: biology, ecology and conservation. Phycologia 45, 5 (2006).
Martin, S. A. & Akin, D. E. Effect of phenolic monomers on the growth and beta-glucosidase activity of Bacteroides ruminicola and on the carboxymethylcellulase, beta-glucosidase, and xylanase activities of Bacteroides succinogenes. Appl. Environ. Microbiol. 54, 3019 (1988).
Dittmar, T., Koch, B., Hertkorn, N. & Kattner, G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol. Oceanogr. 6, 230–235 (2008).
Merder, J. et al. ICBM-OCEAN: processing ultrahigh-resolution mass spectrometry data of complex molecular mixtures. Anal. Chem. 92, 6832–6838 (2020).
Osterholz, H., Kirchman, D. L., Niggemann, J. & Dittmar, T. Environmental drivers of dissolved organic matter molecular composition in the Delaware estuary. Front. Earth Sci. 4, 95 (2016).
Seidel, M. et al. Molecular-level changes of dissolved organic matter along the Amazon river-to-ocean continuum. Mar. Chem. 177, 218–231 (2015).
Agostini, S., Desjobert, J.-M. & Pergent, G. Distribution of phenolic compounds in the seagrass Posidonia oceanica. Phytochemistry 48, 611–617 (1998).
Mateo, M. A., Perez, M., Littler, M. M. & Littler, D. S. Dynamics of millenary organic deposits resulting from the growth of the Mediterranean seagrass Posidonia oceanica. Estuar. Coast. Shelf Sci. 44, 103–110 (1997).
Maie, N., Jaffé, R., Miyoshi, T. & Childers, D. L. Quantitative and qualitative aspects of dissolved organic carbon leached from senescent plants in an oligotrophic wetland. Biogeochemistry 78, 285–314 (2006).
Pind, A., Freeman, C. & Lock, M. A. Enzymic degradation of phenolic materials in peatlands—measurement of phenol oxidase activity. Plant Soil 159, 227–231 (1994).
Fenner, N. & Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 4, 895–900 (2011).
Freeman, C., Ostle, N. & Kang, H. An enzymic ‘latch’ on a global carbon store. Nature 409, 149–149 (2001).
Fenner, N. & Freeman, C. Woody litter protects peat carbon stocks during drought. Nat. Clim. Change 10, 363–369 (2020).
Zhalnina, K. et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 3, 470–480 (2018).
Zwetsloot, M. J., Kessler, A. & Bauerle, T. L. Phenolic root exudate and tissue compounds vary widely among temperate forest tree species and have contrasting effects on soil microbial respiration. New Phytol. 218, 530–541 (2018).
Zwetsloot, M. J. et al. Prevalent root-derived phenolics drive shifts in microbial community composition and prime decomposition in forest soil. Soil Biol. Biochem. 145, 107797 (2020).
Torsvik, V. & Øvreås, L. Microbial diversity and function in soil: from genes to ecosystem. Curr. Opin. Microbiol. 5, 240–245 (2002).
Fitzpatrick, C. R. et al. The plant microbiome: from ecology to reductionism and beyond. Annu. Rev. Microbiol. 74, 81–100 (2020).
Fierer, N. & Jackson, R. B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 103, 626–631 (2006).
Peiffer, J. A. et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 110, 6548 (2013).
O’Banion, B. S., O’Neal, L., Alexandre, G. & Lebeis, S. L. Bridging the gap between single-strain and community-level plant–microbe chemical interactions. Mol. Plant Microbe Interact. 33, 124–134 (2019).
Voges, M. J. E. E. E., Bai, Y., Schulze-Lefert, P. & Sattely, E. S. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc. Natl. Acad. Sci. USA 116, 12558 (2019).
Levin, D. A. Plant phenolics: an ecological perspective. Am. Nat. 105, 157–181 (1971).
Glud, R. N. Oxygen dynamics of marine sediments. Mar. Biol. Res. 4, 243–289 (2008).
Olsen, J. L. et al. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 530, 331–335 (2016).
Calleja, M. L., Marbà, N. & Duarte, C. M. The relationship between seagrass (Posidonia oceanica) decline and sulfide pore water concentration in carbonate sediments. Estuar. Coast. Shelf Sci. 73, 583–588 (2007).
Ramm, A. E. & Bella, D. A. Sulfide production in anaerobic microcosms. Limnol. Oceanogr. 19, 110–118 (1974).
Holmer, M., Andersen, F. Ø., Nielsen, S. L. & Boschker, H. T. S. The importance of mineralization based on sulfate reduction for nutrient regeneration in tropical seagrass sediments. Aquat. Bot. 71, 1–17 (2001).
Kaldy, J. E., Eldridge, P. M., Cifuentes, L. A. & Jones, W. B. Utilization of DOC from seagrass rhizomes by sediment bacteria: 13C-tracer experiments and modeling. Mar. Ecol. Prog. Ser. 317, 41–55 (2006).
Sogin, E. M., Puskas, E., Dubilier, N. & Liebeke, M. Marine metabolomics: a method for the non-targeted measurement of metabolites in seawater by gas-chromatography mass spectrometry. mSystems 4, e00638–19 (2020).
Liebeke, M. & Puskas, E. Drying enhances signal intensities for global GC-MS metabolomics. Metabolites 9, 68 (2019).
Holman, J. D., Tabb, D. L. & Mallick, P. Employing ProteoWizard to convert raw mass spectrometry data. Curr. Protoc. Bioinforma. 46, 11–19 (2014).
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R. & Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78, 779–787 (2006).
Grignon-Dubois, M. & Rezzonico, B. Phenolic fingerprint of the seagrass Posidonia oceanica from four locations in the Mediterranean Sea: first evidence for the large predominance of chicoric acid. Bot. Mar. 58, 379–391 (2015).
Torres, M. E., Mix, A. C. & Rugh, W. D. Precise δ13C analysis of dissolved inorganic carbon in natural waters using automated headspace sampling and continuous-flow mass spectrometry. Limnol. Oceanogr. 3, 349–360 (2005).
Torti, A., Jorgensen, B. B. & Lever, M. A. Preservation of microbial DNA in marine sediments: insights from extracellular DNA pools. Environ. Microbiol. 20, 4526–4542 (2018).
Zhou, J., Bruns, M. A. & Tiedje, J. M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 62, 316–322 (1996).
Lever, M. A. et al. A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types. Front. Microbiol. 6, 476 (2015).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Gruber-Vodicka, H. R., Seah, B. K. B. & Pruesse, E. phyloFlash—rapid small subunit rRNA profiling and targeted assembly from metagenomes. mSystems 5, e00920 (2020).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Nurk, S., Meleshko, D., Korobeynikov, A. & Pevzner, P. A. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 27, 824–834 (2017).
Li, D., Liu, C. M., Luo, R., Sadakane, K. & Lam, T. W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Kang, D. D., Froula, J., Egan, R. & Wang, Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 3, e1165 (2015).
Alneberg, J. et al. Binning metagenomic contigs by coverage and composition. Nat. Methods 11, 1144–1146 (2014).
Wu, Y.-W., Simmons, B. A. & Singer, S. W. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607 (2015).
Sieber, C. M. K. et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat. Microbiol. 3, 836–843 (2018).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Parks, D. H. et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 36, 996–1004 (2018).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 11, 119 (2010).
Zhang, H. et al. dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 46, W95–W101 (2018).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Magrane, M. & UniProt Consortium. UniProt Knowledgebase: a hub of integrated protein data. Database 2011, bar009 (2011).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Finn, R. D. et al. Pfam: the protein families database. Nucleic Acids Res. 42, D222–D230 (2013).
Käll, L., Krogh, A. & Sonnhammer, E. L. L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 338, 1027–1036 (2004).
Huerta-Cepas, J. et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314 (2019).
Kopylova, E., Noé, L. & Touzet, H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28, 3211–3217 (2012).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Lee, M. D. GToTree: a user-friendly workflow for phylogenomics. Bioinformatics 15, 4162–4164 (2019).
Eddy, S. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011).
Edgar, R. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 5, 113 (2004).
Capella-Gutiérrez, S., Silla-Martínez, J. & Gabaldón, T. TrimAl: a tool for automatic alignment trimming. Bioinformatics 25, 1972–1973 (2009).
Nguyen, L., Schmidt, H., Haeseler, A. V. & Minh, B. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259 (2019).
Diepenbroek, M. et al. in Informatik 2014 (eds Plödereder, E. et al.) 1711–1721 (Gesellschaft für Informatik e.V., 2014).
Yilmaz, P. et al. Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications. Nat. Biotech. 29, 415–420 (2011).
Acknowledgements
We thank T. Gulstad, K. Caspersen, M. Weinhold, F. Fojt, J. Beckmann, S. Wetzel and M. Sadowski (Max Planck Institute for Marine Microbiology, MPI-MM) for support with data acquisition and sample preparation. We also thank B. Huettel (Max Planck Genome Center) for his support with sequencing. We are grateful for fruitful discussions with colleagues at the MPI-MM, particularly M. Kuypers, D. de Beer, D. Koopmans, as well as many other colleagues in the Departments of Symbiosis and Biogeochemistry, and T. Dittmar (University Oldenburg). We thank M. Weber, C. Lott and HYDRA staff for sample collections. We are grateful to the Max Planck Society, the Gordon and Betty Moore Foundation (Marine Microbial Initiative Investigator Award to N.D., grant no. GBMF3811) and the MARUM Cluster of Excellence ‘The Ocean Floor’ (Deutsche Forschungsgemeinschaft (Germany Research Foundation) under Germany’s Excellence Strategy EXC-2077-390741603) for financial support. This work is contribution no. 1059 from the Carrie Bow Cay Laboratory, Caribbean Coral Reef Ecosystem Program, National Museum of Natural History, Washington DC.
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
E.M.S., N.D. and M.L. conceived the study. E.M.S. and D.M. collected, processed and analysed primary samples for DOM, metabolomics and sequencing analyses. E.M.S. performed statistical analysis for all primary data. M.S. analysed samples for DOM analysis. S.A. collected and analysed samples for dissolved oxygen concentrations. B.G. collected and analysed MS imaging data. P.B. performed sediment incubation experiments and P.B., S.A. and S.S. analysed data from these incubations. E.M.S., H.G.-V. and D.V.M. designed and optimized bioinformatic pipelines for sequencing analysis. D.V.M. and G.D. reconstructed microbial metabolisms on the basis of sequencing results. G.P. contributed to data interpretation and genomic analyses. E.M.S. wrote the manuscript together with M.L. and N.D., with all other authors contributing to the writing, editing and review of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Marianne Holmer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sugars were more abundant in pore waters underneath P. oceanica than in unvegetated sediments.
A volcano plot comparing gas chromatography–mass spectrometry peaks from pore water metabolites collected inside (In) versus 1 and 20 m away from a seagrass meadow (Edge/Out). Significant peaks (Benjamini-Hochberg adjusted one-way ANOVA p-value < 0.1) are represented by coloured circles (α < 0.1). The grey dashed lines represent Log2-fold changes > 2; The orange dashed line represents Benjamini-Hochberg corrected p-values < 0.1.
Extended Data Fig. 2 Dissolved organic carbon (DOC) concentrations were higher inside the meadow then at the edge or outside.
a, DOC concentrations (Supplementary Table 4) in sediment pore water profiles from inside (n = 6), at the edge (n = 3) and outside (n = 3) of a P. oceanica meadow show DOC concentrations were significantly higher in pore waters inside the meadow and varied as a function of sediment depth (two-way ANOVA p < 0.001; Supplementary Table 2 and Supplementary Table 3). b, A random subset (n = 3) of DOC samples collected inside the meadow show that sugars made up to 40% of the DOC composition within the 5 cm depth, where seagrass roots dominated the sediment.
Extended Data Fig. 3 Sucrose abundances varied across a day/night cycle in plant leaves and sediment pore water.
a, In situ light levels across 24 hours in a Mediterranean P. oceanica meadow off the Island of Elba, Italy (> 2 m water depth). a-d, Violin plots show the relative abundance of sucrose g-1 tissue plant wet weight in b, leaves, c, rhizomes, and d, roots at each sampling time point. Linear models show that sucrose relative abundances were significantly higher in plant leaves at dusk (t = 20:00) than at other times. e, Violin plots showing sucrose concentrations measured in sediment pore waters at each sampling time point. A linear mixed effects model with a random effect of sampling spot shows sucrose concentrations significantly changed throughout the day and were higher during the daylight hours then after midnight (t = 0:00) and before dawn (t = 5:00). The means of each sampling time point (black points) are connected by solid lines. For e, sucrose concentrations greater than the limits of quantification are represented as 200 µM. All statistical results are reported in Supplementary Table 2 and Supplementary Table 3. All data points were transformed before statistical analyses to meet assumptions of normality.
Extended Data Fig. 4 Seagrass roots contained phenolic compounds.
Matrix-assisted laser desorption/ionization mass spectrometry image from a Mediterranean P. oceanica root cross-section collected off the Island of Elba, Italy. The ion image shows the presence of the phenolic compound, identified as caffeic acid (C9H8O4, [M-H]-, m/z 179.0354; Supplementary Fig. 7). Relative ion abundances are visualized from low (blue) to high (yellow).
Extended Data Fig. 5 Composition of sediment microbial communities according to phyla.
a. The relative abundance of taxonomic phyla from each metagenomic library collected inside, at the edge and outside a Mediterranean P. oceanica meadow off the island of Elba, Italy. b. The number of reconstructed MAGs classified by phylum, obtained from the binned co-assembly of all metagenomic reads from these sediments.
Extended Data Fig. 6 Sucrose specialists showed higher expression of genes for metabolizing sucrose than other sugars.
Circle packing plots show the hierarchical relationships between the accumulative expression of glycoside hydrolases (GH) for each MAG from underneath (In), 1 m (Edge) and 20 m (Out) away from a P. oceanica meadow off Elba, Italy. Inner circles are coloured according to the predicted substrates (red = sucrose, light grey = other sugars). The size of the inner circle represents the accumulative transcription level for each MAG (outer white circles) across collection sites. Sucrose specialists are labelled with their MAG numbers.
Supplementary information
Supplementary Information
Supplementary Text 1–4, Methods and Figs. 1–12.
Supplementary Tables
Table 1: Average concentrations of sugars across sampling sites, seagrass species, seasons, times, and depths. Table 2: Statistical results for all models comparing experimental treatments. Table 3: Post-hoc test results signifying differences across models tested in Supplementary Table 2 and associated means tables. Table 4: Dissolved organic carbon and dissolved organic nitrogen concentrations from pore water underneath and adjacent to a P. oceanica meadow. Table 5: Incubation rates of sucrose degradation from seagrass sediments. Table 6: Metagenomic library statistics and accession numbers. Table 7: Metagenomic bin statistics. Table 8: Metatranscriptomic expression data for sucrose specialist MAGs. Table 9: Description of mass spectrometry imaging datasets.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sogin, E.M., Michellod, D., Gruber-Vodicka, H.R. et al. Sugars dominate the seagrass rhizosphere. Nat Ecol Evol 6, 866–877 (2022). https://doi.org/10.1038/s41559-022-01740-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01740-z
This article is cited by
-
Seagrass genomes reveal ancient polyploidy and adaptations to the marine environment
Nature Plants (2024)
-
Visualization of metabolites and microbes at high spatial resolution using MALDI mass spectrometry imaging and in situ fluorescence labeling
Nature Protocols (2023)
-
Microbial associates of an endemic Mediterranean seagrass enhance the access of the host and the surrounding seawater to inorganic nitrogen under ocean acidification
Scientific Reports (2023)
-
Seagrass meadow stability and composition influence carbon storage
Landscape Ecology (2023)
-
A sweet spot in marine ecosystems
Nature Ecology & Evolution (2022)