Abstract
A poor understanding of the fraction of global plant biomass occurring belowground as roots limits our understanding of present and future ecosystem function and carbon pools. Here we create a database of root-mass fractions (RMFs), an index of plant below- versus aboveground biomass distributions, and generate quantitative, spatially explicit global maps of RMFs in trees, shrubs and grasses. Our analyses reveal large gradients in RMFs both across and within vegetation types that can be attributed to resource availability. High RMFs occur in cold and dry ecosystems, while low RMFs dominate in warm and wet regions. Across all vegetation types, the directional effect of temperature on RMFs depends on water availability, suggesting feedbacks between heat, water and nutrient supply. By integrating our RMF maps with existing aboveground plant biomass information, we estimate that in forests, shrublands and grasslands, respectively, 22%, 47% and 67% of plant biomass exists belowground, with a total global belowground fraction of 24% (20–28%), that is, 113 (90–135) Gt carbon. By documenting the environmental correlates of root biomass allocation, our results can inform model projections of global vegetation dynamics under current and future climate scenarios.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The root–shoot ratio data underlying this study are available at https://github.com/haozhima95/Global_mapping_root_shoot_ratio/tree/master/RSR_data. Citations for the root–shoot ratio data are provided in the methods.
Code availability
The code used for this study is available at https://github.com/haozhima95/Global_mapping_root_shoot_ratio.git.
References
Erb, K. H. et al. Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature 553, 73–76 (2018).
Luyssaert, S. et al. Old-growth forests as global carbon sinks. Nature 455, 213–215 (2008).
Drake, J. B. et al. Above-ground biomass estimation in closed canopy Neotropical forests using lidar remote sensing: factors affecting the generality of relationships. Glob. Ecol. Biogeogr. 12, 147–159 (2003).
Lefsky, M. A. et al. Lidar remote sensing of above-ground biomass in three biomes. Glob. Ecol. Biogeogr. 11, 393–399 (2002).
Duncanson, L. et al. The importance of consistent global forest aboveground biomass product validation. Surv. Geophys. 40, 979–999 (2019).
Spawn, S. A., Sullivan, C. C., Lark, T. J. & Gibbs, H. K. Harmonized global maps of above and belowground biomass carbon density in the year 2010. Sci. Data 7, 112 (2020).
Ottaviani, G. et al. The neglected belowground dimension of plant dominance. Trends Ecol. Evol. 35, 763–766 (2020).
Jackson, L. E., Burger, M. & Cavagnaro, T. R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 59, 341–363 (2008).
Gill, R. A. & Jackson, R. B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 147, 13–31 (2000).
Robinson, D. Implications of a large global root biomass for carbon sink estimates and for soil carbon dynamics. Proc. R. Soc. Lond. B 274, 2753–2759 (2007).
Bardgett, R. D., Mommer, L. & De Vries, F. T. Going underground: root traits as drivers of ecosystem processes. Trends Ecol. Evol. 29, 692–699 (2014).
Ribeiro, S. C. et al. Above- and belowground biomass in a Brazilian Cerrado. For. Ecol. Manage. 262, 491–499 (2011).
Mokany, K., Raison, R. J. & Prokushkin, A. S. Critical analysis of root:shoot ratios in terrestrial biomes. Glob. Chang. Biol. 12, 84–96 (2006).
Saatchi, S. S. et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl Acad. Sci. USA 108, 9899–9904 (2011).
Ruesch, A. S. & Gibbs, H. H. K. New IPCC Tier-1 Global Biomass Carbon Map for the Year 2000 (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, 2008).
Chen, J. L. & Reynolds, J. F. A coordination model of whole-plant carbon allocation in relation to water stress. Ann. Bot. 80, 45–55 (1997).
Franklin, O. et al. Modeling carbon allocation in trees: a search for principles. Tree Physiol. 32, 648–666 (2012).
Bloom, A. J., Chapin, F. S. & Mooney, H. A. Resource limitation in plants—an economic analogy. Annu. Rev. Ecol. Syst. 16, 363–392 (1985).
Poorter, H. et al. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30–50 (2012).
Reich, P. in Plant Roots: The Hidden Half (eds. Waisel, Y. et al.) 205–220 (Marcel Dekker, 2006).
Ledo, A. et al. Tree size and climatic water deficit control root to shoot ratio in individual trees globally. New Phytol. 217, 8–11 (2018).
Qi, Y., Wei, W., Chen, C. & Chen, L. Plant root-shoot biomass allocation over diverse biomes: a global synthesis. Glob. Ecol. Conserv. 18, e00606 (2019).
Reich, P. B. et al. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl Acad. Sci. USA 111, 13721–13726 (2014).
De Frenne, P. et al. Latitudinal gradients as natural laboratories to infer species’ responses to temperature. J. Ecol. 101, 784–795 (2013).
Luo, Y. Terrestrial carbon-cycle feedback to climate warming. Annu. Rev. Ecol. Evol. Syst. 38, 683–712 (2007).
Jackson, R. B. et al. A global analysis of root distributions for terrestrial biomes. Oecologia 108, 389–411 (1996).
Malhi, Y., Doughty, C. & Galbraith, D. The allocation of ecosystem net primary productivity in tropical forests. Philos. Trans. R. Soc. Lond. B 366, 3225–3245 (2011).
Roberts, D. R. et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 40, 913–929 (2017).
Cairns, M. A., Brown, S., Helmer, E. H. & Baumgardner, G. A. Root biomass allocation in the world’s upland forests. Oecologia 111, 1–11 (1997).
McCarthy, M. C. & Enquist, B. J. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct. Ecol. 21, 713–720 (2007).
Barton, C. V. M. & Montagu, K. D. Effect of spacing and water availability on root:shoot ratio in Eucalyptus camaldulensis. For. Ecol. Manage. 221, 52–62 (2006).
Enquist, B. J. & Niklas, K. J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 295, 1517–1520 (2002).
Goward, S. N., Tucker, C. J. & Dye, D. G. North American vegetation patterns observed with the NOAA-7 advanced very high resolution radiometer. Vegetatio 64, 3–14 (1985).
Manzoni, S., Jackson, R. B., Trofymow, J. A. & Porporato, A. The global stoichiometry of litter nitrogen mineralization. Science 321, 684–686 (2008).
Kaiser, C., Franklin, O., Dieckmann, U. & Richter, A. Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol. Lett. 17, 680–690 (2014).
Jiao, F., Shi, X. R., Han, F. P. & Yuan, Z. Y. Increasing aridity, temperature and soil pH induce soil C-N-P imbalance in grasslands. Sci. Rep. 6, 19601 (2016).
Sitch, S. et al. Recent trends and drivers of regional sources and sinks of carbon dioxide. Biogeosciences 12, 653–679 (2015).
De Deyn, G. B., Cornelissen, J. H. C. & Bardgett, R. D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 11, 516–531 (2008).
Tjoelker, M. G., Craine, J. M., Wedin, D., Reich, P. B. & Tilman, D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol. 167, 493–508 (2005).
Personeni, E. & Loiseau, P. How does the nature of living and dead roots affect the residence time of carbon in the root litter continuum? Plant Soil 267, 129–141 (2004).
Tuanmu, M. N. & Jetz, W. A global 1-km consensus land-cover product for biodiversity and ecosystem modelling. Glob. Ecol. Biogeogr. 23, 1031–1045 (2014).
Pan, Y., Birdsey, R. A., Phillips, O. L. & Jackson, R. B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 44, 593–622 (2013).
Jackson, R. B., Mooney, H. A. & Schulze, E. D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl Acad. Sci. USA 94, 7362–7366 (1997).
Genet, H., Bréda, N. & Dufrêne, E. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiol. 30, 177–192 (2009).
De Castro, E. A. & Kauffman, J. B. Ecosystem structure in the Brazilian Cerrado: a vegetation gradient of aboveground biomass, root mass and consumption by fire. J. Trop. Ecol. 14, 263–283 (1998).
Ding, B. & Sun, J. Study on biomass of Korean pine plantation in east mountain areas of northeast China. Bull. Bot. Res. 9, 149–157 (1989).
Ding, B., Liu, S. & Cai, T. Studies on biological productivity of artificial forests of Dahurian larches. Chin. J. Plant Ecol. 14, 226–236 (1990).
Ding, B. & Sun, J. Accumulation and distribution of productivity and nutrient element in natural Manchurian ash. J. Northeast For. Univ. 4, 1–9 (1989).
Dossa, E. L., Fernandes, E. C. M., Reid, W. S. & Ezui, K. Above- and belowground biomass, nutrient and carbon stocks contrasting an open-grown and a shaded coffee plantation. Agrofor. Syst. 72, 103–115 (2008).
Epron, D. et al. Do changes in carbon allocation account for the growth response to potassium and sodium applications in tropical Eucalyptus plantations? Tree Physiol. 32, 667–679 (2012).
Fonseca, W., Rey Benayas, J. M. & Alice, F. E. Carbon accumulation in the biomass and soil of different aged secondary forests in the humid tropics of Costa Rica. For. Ecol. Manage. 262, 1400–1408 (2011).
Goodman, R. C. et al. Amazon palm biomass and allometry. For. Ecol. Manage. 310, 994–1004 (2013).
Greenland, D. J. & Kowal, J. M. L. Nutrient content of the moist tropical forest of Ghana. Plant Soil 12, 154–173 (1960).
He, Y. et al. Carbon storage capacity of monoculture and mixed-species plantations in subtropical China. For. Ecol. Manage. 295, 193–198 (2013).
Aiba, M. & Nakashizuka, T. Variation in juvenile survival and related physiological traits among dipterocarp species co‐existing in a Bornean forest. J. Veg. Sci. 18, 379–388 (2007).
Jha, K. K. Carbon storage and sequestration rate assessment and allometric model development in young teak plantations of tropical moist deciduous forest, India. J. For. Res. 26, 589–604 (2015).
Kalita, R. M., Das, A. K. & Nath, A. J. Allometric equations for estimating above- and belowground biomass in Tea (Camellia sinensis (L.) O. Kuntze) agroforestry system of Barak Valley, Assam, northeast India. Biomass Bioenergy 83, 42–49 (2015).
Kenzo, T. et al. Development of allometric relationships for accurate estimation of above- and below-ground biomass in tropical secondary forests in Sarawak, Malaysia. J. Trop. Ecol. 25, 371–386 (2009).
Kenzo, T. et al. Allometric equations for accurate estimation of above-ground biomass in logged-over tropical rainforests in Sarawak, Malaysia. J. For. Res. 14, 365–372 (2009).
Kraenzel, M., Castillo, A., Moore, T. & Potvin, C. Carbon storage of harvest-age teak (Tectona grandis) plantations, Panama. For. Ecol. Manage. 173, 213–225 (2003).
Kuyah, S., Dietz, J., Muthuri, C., van Noordwijk, M. & Neufeldt, H. Allometry and partitioning of above- and below-ground biomass in farmed eucalyptus species dominant in Western Kenyan agricultural landscapes. Biomass Bioenergy 55, 276–284 (2013).
Liu, S., Cai, Y. & Cai, T. in Long-term Research on Forest Ecosystems (ed. Zhou, X.) 419–427 (Northeast Forestry Univ. Press, 1991).
Luo, T. et al. Root biomass along subtropical to alpine gradients: global implication from Tibetan transect studies. For. Ecol. Manage. 206, 349–363 (2005).
Markesteijn, L. & Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J. Ecol. 97, 311–325 (2009).
McNicol, I. M. et al. Development of allometric models for above and belowground biomass in swidden cultivation fallows of northern Laos. For. Ecol. Manage. 357, 104–116 (2015).
Aiba, M. & Nakashizuka, T. Sapling structure and regeneration strategy in 18 Shorea species co-occurring in a tropical rainforest. Ann. Bot. 96, 313–321 (2005).
Menaut, J. C. & Cesar, J. Structure and primary productivity of Lamto savannas, Ivory Coast. Ecology 60, 1197–1210 (1979).
Morais, V. A. et al. Estoques de carbono e biomassa de um fragmento de cerradão em Minas Gerais, Brasil. Cerne 19, 237–245 (2013).
Mugasha, W. A. et al. Allometric models for prediction of above- and belowground biomass of trees in the miombo woodlands of Tanzania. For. Ecol. Manage. 310, 87–101 (2013).
Návar, J. Plasticity of biomass component allocation patterns in semiarid Tamaulipan thornscrub and dry temperate pine species of northeastern Mexico. Polibotánica 31, 121–141 (2011).
Njana, M. A., Eid, T., Zahabu, E. & Malimbwi, R. Procedures for quantification of belowground biomass of three mangrove tree species. Wetl. Ecol. Manage. 23, 749–764 (2015).
Nogueira Junior, L. R., Engel, V. L., Parrotta, J. A., de Melo, A. C. G. & Ré, D. S. Equações alométricas para estimativa da biomassa arbórea em plantios mistos com espécies nativas na restauração da Mata Atlântica. Biota Neotrop. 14, 1–9 (2014).
Peichl, M. & Arain, M. A. Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 140, e20130084 (2006).
Battles, J. J. et al. Vegetation composition, structure, and biomass of two unpolluted watersheds in the Cordillera de Piuchué, Chiloé Island, Chile. Plant Ecol. 158, 5–19 (2002).
Ryan, C. M., Williams, M. & Grace, J. Above- and belowground carbon stocks in a miombo woodland landscape of Mozambique. Biotropica 43, 423–432 (2011).
Saint-André, L. et al. Age-related equations for above- and below-ground biomass of a Eucalyptus hybrid in Congo. For. Ecol. Manage. 205, 199–214 (2005).
Aryal, D. R., De Jong, B. H. J., Ochoa-Gaona, S., Esparza-Olguin, L. & Mendoza-Vega, J. Carbon stocks and changes in tropical secondary forests of southern Mexico. Agric. Ecosyst. Environ. 195, 220–230 (2014).
Schepaschenko, D. et al. A dataset of forest biomass structure for Eurasia. Sci. Data 4, 170070 (2017).
Schroth, G., D’Angelo, S. A., Teixeira, W. G., Haag, D. & Lieberei, R. Conversion of secondary forest into agroforestry and monoculture plantations in Amazonia: consequences for biomass, litter and soil carbon stocks after 7 years. For. Ecol. Manage. 163, 131–150 (2002).
Schulze, E. D. et al. Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 108, 503–511 (1996).
Stolbovoi, V. & McCallum, I. Land resources of Russia [CD] (International Institute for Applied Systems Analysis and the Russian Academy of Science, 2002); http://www.iiasa.ac.at/Research/FOR/russia_cd/guide.htm
Wang, L. et al. Biomass allocation patterns across China’s terrestrial biomes. PLoS ONE 9, e93566 (2014).
Wauters, J. B., Coudert, S., Grallien, E., Jonard, M. & Ponette, Q. Carbon stock in rubber tree plantations in Western Ghana and Mato Grosso (Brazil). For. Ecol. Manage. 255, 2347–2361 (2008).
Williams-Linera, G. Biomass and nutrient content in two successional stages of tropical wet forest in Uxpanapa, Mexico. Biotropica 15, 275–284 (1983).
Xu, Y. et al. Improving allometry models to estimate the above- and belowground biomass of subtropical forest, China. Ecosphere 6, 289 (2015).
Youkhana, A. H. & Idol, T. W. Allometric models for predicting above- and belowground biomass of Leucaena-KX2 in a shaded coffee agroecosystem in Hawaii. Agrofor. Syst. 83, 331–345 (2011).
Zhang, H. et al. Biogeographical patterns of biomass allocation in leaves, stems, and roots in China’s forests. Sci. Rep. 5, 15997 (2015).
Castellanos, J., Maass, M. & Kummerow, J. Root biomass of a dry deciduous tropical forest in Mexico. Plant Soil 131, 225–228 (1991).
Zheng, Z., Feng, Z., Cao, M., Li, Z. & Zhang, J. Forest structure and biomass of a tropical seasonal rain forest in Xishuangbanna, southwest China. Biotropica 38, 318–327 (2006).
Návar, J. Root stock biomass and productivity assessments of reforested pine stands in northern Mexico. For. Ecol. Manage. 338, 139–147 (2015).
Wang, X., Fang, J. & Zhu, B. Forest biomass and root–shoot allocation in northeast China. For. Ecol. Manage. 255, 4007–4020 (2008).
Chen, D. K., Zhou, X. F., Zhao, H. X., Wang, Y. H. & Jing, Y. Y. Study on the structure, function and succession of the four types in natural secondary forest. J. Northeast For. Univ. 2, 1–20 (1982).
Chidumayo, E. N. Estimating tree biomass and changes in root biomass following clear-cutting of Brachystegia-Julbernardia (miombo) woodland in central Zambia. Environ. Conserv. 41, 54–63 (2014).
Coll, L., Potvin, C., Messier, C. & Delagrange, S. Root architecture and allocation patterns of eight native tropical species with different successional status used in open-grown mixed plantations in Panama. Trees 22, 585–596 (2008).
Das, D. K. & Chaturvedi, O. P. Structure and function of Populus deltoides agroforestry systems in eastern India: 1. dry matter dynamics. Agrofor. Syst. 65, 215–221 (2005).
Ni, J. Estimating net primary productivity of grasslands from field biomass measurements in temperate northern China. Plant Ecol. 174, 217–234 (2011).
Olson, R. et al. NPP Multi-Biome: Summary Data from Intensive Studies at 125 Sites, 1936–2006 (ORNL DAAC, accessed 19 June 2019); https://daac.ornl.gov/cgi-bin/dsviewer.pl?ds_id=1352
Perez, C. A. & Frangi, J. L. Grassland biomass dynamics along an altitudinal gradient in the pampa. J. Range Manage. 53, 518–528 (2007).
Perez-Quezada, J. F. F., Delpiano, C. A. A., Snyder, K. A. A., Johnson, D. A. A. & Franck, N. Carbon pools in an arid shrubland in Chile under natural and afforested conditions. J. Arid Environ. 75, 29–37 (2011).
Pornon, A., Boutin, M. & Lamaze, T. Contribution of plant species to the high N retention capacity of a subalpine meadow undergoing elevated N deposition and warming. Environ. Pollut. 245, 235–242 (2019).
Ramakrishnan, P. S. & Ram, S. C. Vegetation, biomass and productivity of seral grasslands of Cherrapunji in north-east India. Vegetatio 74, 47–53 (1988).
Shaver, G. R., Laundre, J. A., Giblin, A. E. & Nadelhoffer, K. J. Changes in live plant biomass, primary production, and species composition along a riverside toposequence in Arctic Alaska, USA. Arct. Alp. Res. 28, 363–379 (2006).
Smith, J. M. B. & Klinger, L. F. Aboveground:belowground phytomass ratios in Venezuelan paramo vegetation and their significance. Arct. Alp. Res. 17, 189–198 (2006).
Sun, J. et al. Effects of grazing regimes on plant traits and soil nutrients in an alpine steppe, northern Tibetan Plateau. PLoS ONE 9, e108821 (2014).
Wang, P. et al. Belowground plant biomass allocation in tundra ecosystems and its relationship with temperature. Environ. Res. Lett. 11, 055003 (2016).
Yang, Y., Fang, J., Ji, C. & Han, W. Above- and belowground biomass allocation in Tibetan grasslands. J. Veg. Sci. 20, 177–184 (2009).
Yang, Y., Fang, J., Ma, W., Guo, D. & Mohammat, A. Large-scale pattern of biomass partitioning across China’s grasslands. Glob. Ecol. Biogeogr. 19, 268–277 (2010).
Geng, H. L., Wang, Y. H., Wang, F. Y. & Jia, B. R. The dynamics of root-shoot ratio and its environmental effective factors of recovering Leymus chinensis steppe vegetation in Inner Mongolia, China. Acta Ecol. Sin. 28, 4629–4634 (2008).
Hui, D. & Jackson, R. B. Geographical and interannual variability in biomass partitioning in grassland ecosystems: a synthesis of field data. New Phytol. 169, 85–93 (2006).
Jouquet, P., Tavernier, V., Abbadie, L. & Lepage, M. Nests of subterranean fungus-growing termites (Isoptera, Macrotermitinae) as nutrient patches for grasses in savannah ecosystems. Afr. J. Ecol. 43, 191–196 (2005).
Leonid, U. et al. Impact of climate and grazing on biomass components of eastern Russia typical steppe. J. Integr. Agric. 13, 1183–1192 (2014).
Lucash, M. S., Farnsworth, B. & Winner, W. E. Response of sagebrush steppe species to elevated CO2 and soil temperature. West. N. Am. Nat. 65, 80–86 (2005).
Luo, W. et al. Patterns of plant biomass allocation in temperate grasslands across a 2500-km transect in northern China. PLoS ONE 8, e71749 (2013).
Barbour, M. G. Desert dogma reexamined: root/shoot productivity and plant spacing. Am. Midl. Nat. 89, 41–57 (1973).
Becker, P., Sharbini, N. & Yahya, R. Root architecture and root:shoot allocation of shrubs and saplings in two lowland tropical forests: implications for life-form composition. Biotropica 31, 93–101 (1999).
Becker, P. & Castillo, A. Root architecture of shrubs and saplings in the understory of a tropical moist forest in lowland Panama. Biotropica 22, 242–249 (1990).
Beier, C. et al. Carbon and nitrogen balances for six shrublands across Europe. Glob. Biogeochem. Cycles 23, GB4008 (2009).
Bhatt, Y. D., Rawat, Y. S. & Singh, S. P. Changes in ecosystem functioning after replacement of forest by Lantana shrubland in Kumaun Himalaya. J. Veg. Sci. 5, 67–70 (1994).
Caldwell, M. M., White, R. S., Moore, R. T. & Camp, L. B. Carbon balance, productivity, and water use of cold-winter desert shrub communities dominated by C3 and C4 species. Oecologia 29, 275–300 (1977).
De Viñas, I. C. R. et al. Biomass of root and shoot systems of Quercus coccifera shrublands in eastern Spain. Ann. For. Sci. 57, 803–810 (2000).
Caravaca, F., Figueroa, D., Alguacil, M. M. & Roldán, A. Application of composted urban residue enhanced the performance of afforested shrub species in a degraded semiarid land. Bioresour. Technol. 90, 65–70 (2003).
Caravaca, F., Figueroa, D., Azcón-Aguilar, C., Barea, J. M. & Roldán, A. Medium-term effects of mycorrhizal inoculation and composted municipal waste addition on the establishment of two Mediterranean shrub species under semiarid field conditions. Agric. Ecosyst. Environ. 97, 95–105 (2003).
Carrasco, L., Azcón, R., Kohler, J., Roldán, A. & Caravaca, F. Comparative effects of native filamentous and arbuscular mycorrhizal fungi in the establishment of an autochthonous, leguminous shrub growing in a metal-contaminated soil. Sci. Total Environ. 409, 1205–1209 (2011).
Carrillo-Garcia, Á., Bashan, Y. & Bethlenfalvay, G. J. Resource-island soils and the survival of the giant cactus, cardon, of Baja California Sur. Plant Soil 218, 207–214 (2000).
Carrión-Prieto, P. et al. Mediterranean shrublands as carbon sinks for climate change mitigation: new root-to-shoot ratios. Carbon Manage. 8, 67–77 (2017).
Deng, L., Han, Q. S., Zhang, C., Tang, Z. S. & Shangguan, Z. P. Above-ground and below-ground ecosystem biomass accumulation and carbon sequestration with Caragana korshinskii Kom plantation development. Land Degrad. Dev. 28, 906–917 (2017).
Perkins, S. R. & Owens, M. K. Growth and biomass allocation of shrub and grass seedlings in response to predicted changes in precipitation seasonality. Plant Ecol. 168, 107–120 (2003).
Gargaglione, V., Peri, P. L. & Rubio, G. Allometric relations for biomass partitioning of Nothofagus antarctica trees of different crown classes over a site quality gradient. For. Ecol. Manage. 259, 1118–1126 (2010).
Hao, H. M. et al. Effects of shrub patch size succession on plant diversity and soil water content in the water-wind erosion crisscross region on the Loess Plateau. Catena 144, 177–183 (2016).
Herwitz, S. R. & Olsvig-Whittaker, L. Preferential upslope growth of Zygophyllum dumosum Boiss. (Zygophyllaceae) roots into bedrock fissures in the northern Negev desert. J. Biogeogr. 16, 457–460 (1989).
Hoffmann, A. & Kummerow, J. Root studies in the Chilean matorral. Oecologia 32, 57–69 (1978).
Holl, K. D. Effects of above- and below-ground competition of shrubs and grass on Calophyllum brasiliense (Camb.) seedling growth in abandoned tropical pasture. For. Ecol. Manage. 109, 187–195 (1998).
Hollister, R. D. & Flaherty, K. J. Above- and below-ground plant biomass response to experimental warming in northern Alaska. Appl. Veg. Sci. 13, 378–387 (2010).
Kizito, F. et al. Seasonal soil water variation and root patterns between two semi-arid shrubs co-existing with pearl millet in Senegal, West Africa. J. Arid Environ. 67, 436–455 (2006).
Kummerow, J., Krause, D. & Jow, W. Root systems of chaparral shrubs. Oecologia 29, 163–177 (1977).
León, M. F., Squeo, F. A., Gutiérrez, J. R. & Holmgren, M. Rapid root extension during water pulses enhances establishment of shrub seedlings in the Atacama Desert. J. Veg. Sci. 22, 120–129 (2011).
Li, C. P. & Xiao, C. W. Above- and belowground biomass of Artemisia ordosica communities in three contrasting habitats of the Mu Us Desert, northern China. J. Arid Environ. 70, 195–207 (2007).
Liang, Y. M., Hazlett, D. L. & Lauenroth, W. K. Biomass dynamics and water use efficiencies of five plant communities in the shortgrass steppe. Oecologia 80, 148–153 (1989).
Zan, Q., Wang, Y., Liao, B. & Zheng, D. Biomass and net productivity of Sonneratia apetala, S. caseolaris mangrove man-made forest. Wuhan Bot. Res. 19, 391–396 (2001).
Liao, B., Zheng, D. & Zheng, S. Studies on the biomass of Sonneratia caseolaris stand. For. Res. 3, 47–54 (1990).
Lufafa, A. et al. Allometric relationships and peak-season community biomass stocks of native shrubs in Senegal’s Peanut Basin. J. Arid Environ. 73, 260–266 (2009).
Lusk, C. H. Leaf area and growth of juvenile temperate evergreens in low light: species of contrasting shade tolerance change rank during ontogeny. Funct. Ecol. 18, 820–828 (2004).
Marsh, A. S., Arnone, J. A., Bormann, B. T. & Gordon, J. C. The role of Equisetum in nutrient cycling in an Alaskan shrub wetland. J. Ecol. 88, 999–1011 (2000).
Martínez, F. et al. Belowground structure and production in a Mediterranean sand dune shrub community. Plant Soil 201, 209–216 (1998).
Marziliano, P. A. et al. Estimating belowground biomass and root/shoot ratio of Phillyrea latifolia L. in the Mediterranean forest landscapes. Ann. For. Sci. 72, 585–593 (2015).
Mauchamp, A., Montaña, C., Lepart, J., Rambal, S. & Montana, C. Ecotone dependent recruitment of a desert shrub, Flourensia cernua, in vegetation stripes. Oikos 68, 107–116 (1993).
Mendoza-Ponce, A. & Galicia, L. Aboveground and belowground biomass and carbon pools in highland temperate forest landscape in central Mexico. Forestry 83, 497–506 (2010).
Miller, P. C. & Ng, E. Root:shoot biomass ratios in shrubs in southern California and central Chile. Madrono 24, 215–223 (1977).
Mooney, H. A. & Rundel, P. W. Nutrient relations of the evergreen shrub, Adenostoma fasciculatum, in the California chaparral. Bot. Gaz. 140, 109–113 (1979).
Moro, M. J., Pugnaire, F. I., Haase, P. & Puigdefábregas, J. Effect of the canopy of Retama sphaerocarpa on its understorey in a semiarid environment. Funct. Ecol. 11, 425–431 (1997).
Negreiros, D., Fernandes, G. W., Silveira, F. A. O. & Chalub, C. Seedling growth and biomass allocation of endemic and threatened shrubs of rupestrian fields. Acta Oecol. 35, 301–310 (2009).
Nie, X., Yang, Y., Yang, L. & Zhou, G. Above- and belowground biomass allocation in shrub biomes across the northeast Tibetan Plateau. PLoS ONE 11, e0154251 (2016).
Nobel, P. S., Quero, E. & Linares, H. Root versus shoot biomass: responses to water, nitrogen, and phosphorus applications for Agave lechuguilla. Bot. Gaz. 150, 411–416 (1989).
Pacaldo, R. S., Volk, T. A. & Briggs, R. D. Greenhouse gas potentials of shrub willow biomass crops based on below- and aboveground biomass inventory along a 19-year chronosequence. Bioenergy Res. 6, 252–262 (2013).
Padilla, F. M., Miranda, J. D., Jorquera, M. J. & Pugnaire, F. I. Variability in amount and frequency of water supply affects roots but not growth of arid shrubs. Plant Ecol. 204, 261–270 (2009).
Portsmuth, A., Niinemets, Ü., Truus, L. & Pensa, M. Biomass allocation and growth rates in Pinus sylvestris are interactively modified by nitrogen and phosphorus availabilities and by tree size and age. Can. J. For. Res. 35, 2346–2359 (2005).
Roth, G. A., Whitford, W. G. & Steinberger, Y. Jackrabbit (Lepus californicus) herbivory changes dominance in desertified Chihuahuan Desert ecosystems. J. Arid Environ. 70, 418–426 (2007).
Ruiz-Peinado, R., Moreno, G., Juarez, E., Montero, G. & Roig, S. The contribution of two common shrub species to aboveground and belowground carbon stock in Iberian dehesas. J. Arid Environ. 91, 22–30 (2013).
Rundel, P. W. Biomass, productivity, and nutrient allocation in subalpine shrublands and meadows of the Emerald Lake Basin, Sequoia National Park, California. Arct. Antarct. Alp. Res. 47, 115–123 (2015).
Millikin, C. S. & Bledsoe, C. S. Biomass and distribution of fine and coarse roots from blue oak (Quercus douglasii) trees in the northern Sierra Nevada foothills of California. Plant Soil 214, 27–38 (1999).
Saura-Mas, S. & Lloret, F. Adult root structure of Mediterranean shrubs: relationship with post-fire regenerative syndrome. Plant Biol. 16, 147–154 (2014).
Schenk, H. J. & Mahall, B. E. Positive and negative plant interactions contribute to a north-south-patterned association between two desert shrub species. Oecologia 132, 402–410 (2002).
Silva, J. S., Rego, F. C. & Martins-Loução, M. A. Belowground traits of Mediterranean woody plants in a Portuguese shrubland. Ecol. Mediterr. 28, 5–13 (2002).
Simões, M. P., Madeira, M. & Gazarini, L. Biomass and nutrient dynamics in Mediterranean seasonal dimorphic shrubs: strategies to face environmental constraints. Plant Biosyst. 146, 500–510 (2012).
Tao, Y., Zhang, Y. M. & Downing, A. Similarity and difference in vegetation structure of three desert shrub communities under the same temperate climate but with different microhabitats. Bot. Stud. 54, 59 (2013).
Toscano, S., Scuderi, D., Giuffrida, F. & Romano, D. Responses of Mediterranean ornamental shrubs to drought stress and recovery. Sci. Hortic. 178, 145–153 (2014).
Trubat, R., Cortina, J. & Vilagrosa, A. Nutrient deprivation improves field performance of woody seedlings in a degraded semi-arid shrubland. Ecol. Eng. 37, 1164–1173 (2011).
Van Wijk, M. T., Williams, M., Gough, L., Hobbie, S. E. & Shaver, G. R. Luxury consumption of soil nutrients: a possible competitive strategy in above-ground and below-ground biomass allocation and root morphology for slow-growing arctic vegetation? J. Ecol. 91, 664–676 (2003).
Walker, L. R., Clarkson, B. D., Silvester, W. B. & Clarkson, B. R. Colonization dynamics and facilitative impacts of a nitrogen-fixing shrub in primary succession. J. Veg. Sci. 14, 277–290 (2003).
Wang, B. & Yang, X. S. Comparison of biomass and species diversity of four typical zonal vegetations. J. Fujian Coll. For. 29, 345–350 (2009).
Wang, M. & Li, H. Quantitative study on the soil water dynamics of various forest plantations in the Loess Plateau region in northwestern Shanxi. Acta Ecol. Sin. 2, 178–184 (1995).
Wang, P. et al. Seasonal changes and vertical distribution of root standing biomass of graminoids and shrubs at a Siberian tundra site. Plant Soil 407, 55–65 (2016).
Whittaker, R. H. & Woodwell, G. M. Dimension and production relations of trees and shrubs in the Brookhaven Forest, New York. J. Ecol. 56, 1–25 (1968).
Xu, H., Li, Y., Xu, G. & Zou, T. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant Cell Environ. 30, 399–409 (2007).
Yan, Z. Biomass and its allocation in a 28-year-old Castanopsis kawakamii plantation. J. Fujian Coll. For. 2, 114–118 (1996).
Gong, Y. et al. Carbon storage and vertical distribution in three shrubland communities in Gurbantünggüt Desert, Uygur Autonomous Region of Xinjiang, northwest China. Chin. Geogr. Sci. 22, 541–549 (2012).
Yu, Y., Shi, D., Qiuyi, J., He, L. & Cheng, G. On the biomass of secondary Schima superba forest in Hangzhou. J. Zhejiang For. Coll. 2, 157–161 (1993).
Kato, T. et al. Carbon dioxide exchange between the atmosphere and an alpine meadow ecosystem on the Qinghai-Tibetan Plateau, China. Agric. Meteorol. 124, 121–134 (2004).
Li, Z., Zhu, Q. & Li, J. A comparison of photosynthetic carbon sequestration of four shrubs in Ningxia. Pratacultural Sci. 29, 352–357 (2012).
Zhu, X., Shi, Q. & Li, Y. A preliminary study on the Qinghai’s treasure house of the forest biomass and shrubs. Sci. Technol. Qinghai Agric. For. 1, 15–20 (1993).
Liao, B. & Zheng, D. Study on the forest biomass and productivity of olive wood. For. Res. 4, 22–29 (1991).
Liu, B., Liu, Z., Lü, X., Maestre, F. T. & Wang, L. Sand burial compensates for the negative effects of erosion on the dune-building shrub Artemisia wudanica. Plant Soil 374, 263–273 (2014).
Alguacil, M. M., Hernández, J. A., Caravaca, F., Portillo, B. & Roldán, A. Antioxidant enzyme activities in shoots from three mycorrhizal shrub species afforested in a degraded semi-arid soil. Physiol. Plant. 118, 562–570 (2003).
Axe, M. S., Grange, I. D. & Conway, J. S. Carbon storage in hedge biomass—a case study of actively managed hedges in England. Agric. Ecosyst. Environ. 250, 81–88 (2017).
van den Hoogen, J. et al. Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198 (2019).
Erin, L. et al. h2o: R Interface for the ‘H2O’ Scalable Machine Learning Platform. R package v.3.32.0.2 (2020); https://github.com/h2oai/h2o-3
Sagi, O. & Rokach, L. Ensemble learning: a survey. WIREs Data Min. Knowl. Discov. 8, e1249 (2018).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Gorelick, N. et al. Google Earth Engine: planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27 (2017).
Heiberger, R. M. HH: Statistical Analysis and Data Display: Heiberger and Holland (2020).
Hothorn, T. & Zeileis, A. partykit: A modular toolkit for recursive partytioning in R. J. Mach. Learn. Res. 16, 3905–3909 (2015).
Borkovec, M. & Madin, N. ggparty: ‘ggplot’ visualizations for the ‘partykit’ package (2019).
Dormann, C. F. Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Glob. Ecol. Biogeogr. 16, 129–138 (2007).
Hutchinson, M., Xu, T., Houlder, D., Nix, H. & McMahon, J. ANUCLIM 6.0 User’s Guide (Australian National Univ., 2009).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Global Aridity and PET database (CGIAR-CSI, accessed 15 May 2018); http://www.cgiarcsi.community/data/global-aridity-and-pet-database
CIESIN Gridded Population of the World, version 4 (GPWv4): Population Density Adjusted to Match 2015 Revision UN WPP Country Totals (NASA SEDAC, 2018); https://doi.org/10.7927/H4HX19NJ
Venter, O. et al. Global terrestrial human footprint maps for 1993 and 2009. Sci. Data 3, 160067 (2016).
SoilGrids (ISRIC, accessed 15 May 2018); https://www.soilgrids.org
Entekhabi, D. et al. The soil moisture active passive (SMAP) mission. Proc. IEEE 98, 704–716 (2010).
Fan, Y., Li, H. & Miguez-Macho, G. Global patterns of groundwater table depth. Science 339, 940–943 (2013).
Batjes, N. H. Harmonized soil property values for broad-scale modelling (WISE30sec) with estimates of global soil carbon stocks. Geoderma 269, 61–68 (2016).
Schaaf, C. & Wang, Z. MCD43A1 MODIS/Terra+Aqua BRDF/Albedo Model Parameters Daily L3 Global - 500m V006 (NASA LP DAAC, 2015); https://doi.org/10.5067/MODIS/MCD43A1C.006
Didan, K. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid V006 (NASA LP DAAC, 2015).
Crowther, T. W. et al. Mapping tree density at a global scale. Nature 525, 201–205 (2015).
Acknowledgements
We thank J.-F. Bastin, P. B. Reich, R. B. Jackson and Y. Zeng for their constructive comments on this study. This work was supported by grants to C.M.Z. from the ETH Zurich Postdoctoral Fellowship programme, L.M. from the China Scholarship Council and T.W.C. from DOB Ecology. B.D.S. was funded by the Swiss National Science Foundation grant no. PCEFP2_181115. C.T. was supported by a Lawrence Fellow award through the Lawrence Livermore National Laboratory, the US Department of Energy under contract DE-AC52-07NA27344 and the Lawrence Livermore National Laboratory LDRD (Laboratory Directed Research & Development) Program under project no. 20-ERD-055.
Author information
Authors and Affiliations
Contributions
H.M., L.M., T.W.C. and C.M.Z. conceived and developed the study and wrote the manuscript. H.M. and L.M. collected the data. H.M. and L.M. performed the analyses. D.S.M., J.v.d.H., B.S. and C.T. gave input on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Ecology & Evolution thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
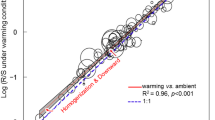
Extended Data Fig. 2 Random 10-fold cross-validation (RCV) of the spatial root-mass fraction models and spatial autocorrelation of model residuals.
a–c, Heat plots showing the relationships between predicted and observed RMFs in forests (a), shrublands (b), and grasslands (c) based on RCV. Solid lines indicate fitted relationships based on ordinary least squares regression [coefficient of determination values relative to the 1:1 line (equation 2) shown in the bottom right corner], dashed diagonal lines indicate a 1:1 relationship between observed and predicted points. d–f, The standard errors of the observed (black) and predicted (grey) mean values of root mass fractions decrease with increasing sample size. The operation was repeated with 1,000 random seeds for the observed and predicted mean values, and the calculated standard errors of the mean are shown. Note, ‘sample size’ in D–F refers to the number of pixels, and thus denotes square kilometres. g–i, Semivariograms illustrating spatial autocorrelation of model residuals in forests (g), shrublands (h) and grasslands (i). Semivariances of residuals were computed based on random 10-fold cross validation (blue) and spatial leave-one-out cross-validation (LOO-CV) with buffer radii of 150km (dark green), 250km (green) and 500km (light green). Dashed vertical lines indicate the buffer radii of the final validation model reported throughout the text.
Extended Data Fig. 3 Partial regression coefficients for the effects of 8 environmental covariates from linear multiple regression models.
To reduce the influence of spatial autocorrelation, a bootstrapping procedure was applied for the forest data (see Methods). Red dots indicate positive effects on RMFs, blue dots indicate negative effects. Error bars reflect two standard errors either side of the mean partial regression coefficient.
Extended Data Fig. 4 Recursive partitioning trees for the univariate effects of annual mean temperature (a), soil moisture (b), NDVI (c), and sand content (d) on RMFs in forests.
These four variables were chosen on basis of the random forest variable importance metric (Fig. 3a) and, for each model, the remaining three variables were evaluated as potential split points. The number of independent observations contained in each terminal node was constrained to ≥10% of the total data (500 observations). Regression plots show slopes and 95% confidence intervals.
Extended Data Fig. 5 Recursive partitioning trees for the univariate effects of annual mean temperature (a), soil moisture (b), aridity index (c), and NDVI (d) on RMFs in shrublands.
These four variables were chosen on basis of the random forest variable importance metric (Fig. 3b) and, for each model, the remaining three variables were evaluated as potential split points. The number of independent observations contained in each terminal node was constrained to ≥10% of the total data (30 observations). Regression plots show slopes and 95% confidence intervals.
Extended Data Fig. 6 Recursive partitioning trees for the univariate effects of annual mean temperature (a), soil moisture (b), aridity index (c), and NDVI (d) on RMFs in grasslands.
These four variables were chosen on basis of the random forest variable importance metric (Fig. 3c) and, for each model, the remaining three variables were evaluated as potential split points. The number of independent observations contained in each terminal node was constrained to ≥10% of the total data (120 observations). Regression plots show slopes and 95% confidence intervals.
Extended Data Fig. 7 Comparison of observed forest RMFs with predicted RMFs from dynamic global vegetation models and a current-generation biomass map.
The blue bars represent histograms of predicted RMF values based on our LOO-CV procedure (a), current-generation biomass estimates6 (b), and the vegetation models CABLE-POP (c), CLASS-CTEM (d), ISAM (e) and ORCHIDEE (f). Yellow bars represent observed values. Insets show scatter plots of predicted versus observed RMFs with solid lines indicating fitted relationships, dashed diagonal lines indicating a 1:1 relationship between observed and predicted points. For the vegetation models, forest was defined as pixels with a tree cover fraction higher than 50%.
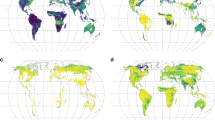
Extended Data Fig. 8 The global distribution of belowground plant biomass and associated uncertainties in forests (a, b), grasslands (c, d), and shrublands (e, f).
a, c, e, Belowground plant biomass (in tons carbon per hectare). b, d, f Associated uncertainties in belowground carbon, calculated as the predicted biomass range (based on 2.5% and 97.5% RMF quantiles derived from the bootstrapped RMF models) divided by the mean predicted biomass in each pixel. Maps are projected at 30 arc-seconds (~1 km2) resolution.
Extended Data Fig. 9 Root mass fraction inter-model consistency in forests (a), grasslands (b) and shrublands (c).
Inter-model consistency was calculated as the coefficient of variation (standard deviation divided by mean, in %) of the predictions of the 10 best models. Maps are projected at 30 arc-seconds (~1 km2) resolution.
Extended Data Fig. 10 The extent of interpolation and extrapolation across all terrestrial pixels in which the respective vegetation type, forest (a), grassland (b) and shrubland (c) occurs.
Values represent the percentage of interpolation based on principal component analysis, that is, the percentage of bands that fall into the convex hull space.
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, H., Mo, L., Crowther, T.W. et al. The global distribution and environmental drivers of aboveground versus belowground plant biomass. Nat Ecol Evol 5, 1110–1122 (2021). https://doi.org/10.1038/s41559-021-01485-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-021-01485-1
This article is cited by
-
Historical impacts of grazing on carbon stocks and climate mitigation opportunities
Nature Climate Change (2024)
-
Vegetation–environment interactions: plant species distribution and community assembly in mixed coniferous forests of Northwestern Himalayas
Scientific Reports (2023)
-
High precipitation rates increase potassium density in plant communities in the Tibetan Plateau
Communications Earth & Environment (2023)
-
Climate-driven ecological thresholds in China’s drylands modulated by grazing
Nature Sustainability (2023)
-
Global Positive Effects of Litter Inputs on Soil Nitrogen Pools and Fluxes
Ecosystems (2023)