Abstract
Accumulating behavioural data indicate that aggregation pheromones may mediate the formation and maintenance of mosquito swarms. However, chemical cues possibly luring mosquitoes to swarms have not been adequately investigated, and the likely molecular incitants of these complex reproductive behaviours remain unknown. Here we show that males of the important malaria vector species Anopheles arabiensis and An. gambiae produce and release aggregation pheromones that attract individuals to the swarm and enhance mating success. We found that males of both species released significantly higher amounts of 3-hydroxy-2-butanone (acetoin), 6-methyl-5-hepten-2-one (sulcatone), octanal, nonanal and decanal during swarming in the laboratory. Feeding males with stable-isotope-labelled glucose revealed that the males produced these five compounds. A blend composed of synthetic analogues to these swarming odours proved highly attractive to virgin males and females of both species under laboratory conditions and substantially increased mating in five African malaria vectors (An. gambiae, An. coluzzii, An. arabiensis, An. merus and An. funestus) in semi-field experiments. Our results not only narrow a conspicuous gap in understanding a vital aspect of the chemical ecology of male mosquitoes but also demonstrate fundamental roles of rhythmic and metabolic genes in the physiology and behavioural regulation of these vectors. These identified aggregation pheromones have great potential for exploitation against these highly dangerous insects. Manipulating such pheromones could increase the efficacy of malaria-vector control programmes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The sequencing data are available at the GEO database (SRA accession no. PRJNA590389). The data presented in this publication have been deposited in the National Centre for Biotechnology’s Gene Expression Omnibus and are accessible through GEO series accession no. PRJNA590389. The raw data, including the amounts of swarming odours, are presented in Supplementary Table 3a–e.
References
Clements, A. N. The Biology of Mosquitoes: Sensory Reception and Behaviour Vol. 2 (CAB1 Publishing, 1999).
Diabaté, A. et al. Spatial distribution and male mating success of Anopheles gambiae swarms. BMC Evol. Biol. 11, 184 (2011).
Assogba, B. S. et al. Characterization of swarming and mating behaviour between Anopheles coluzzii and Anopheles melas in a sympatry area of Benin. Acta Trop. 132, S53–S63 (2014).
Sawadogo, P. S. et al. Swarming behaviour in natural populations of Anopheles gambiae and An. coluzzii: review of 4 years survey in rural areas of sympatry, Burkina Faso (West Africa). Acta Trop. 132, S42–S52 (2014).
Zawada, J. W. et al. Molecular and physiological analysis of Anopheles funestus swarms in Nchelenge, Zambia. Malar. J. 17, 49 (2018).
Achinko, D. et al. Swarming and mating activity of Anopheles gambiae mosquitoes in semi-field enclosures. Med. Vet. Entomol. 30, 14–20 (2016).
Kaindoa, E. W. et al. Swarms of the malaria vector Anopheles funestus in Tanzania. Malar. J. 18, 29 (2019).
Lees, R. S. et al. Review: improving our knowledge of male mosquito biology in relation to genetic control programmes. Acta Trop. 132, S2–S11 (2013).
Howell, P. I. & Knols, B. G. J. Male mating biology. Malar. J. 8, S8 (2009).
Jones, M. D. R., Gubbins, S. J. & Cubbin, C. M. Circadian flight activity in 4 sibling species of Anopheles-gambiae complex (Diptera, Culicidae). Bull. Entomol. Res. 64, 241–246 (1974).
Rund, S. S. C., Lee, S. J., Bush, B. R. & Duffield, G. E. Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J. Insect Physiol. 58, 1609–1619 (2012).
Charlwood, J. D. & Jones, M. D. R. Mating in the mosquito, Anopheles-gambiae s-l. II. Swarming behavior. Physiol. Entomol. 5, 315–320 (1980).
Niang, A. et al. Semi-field and indoor setups to study malaria mosquito swarming behavior. Parasit. Vectors 12, 446 (2019).
Marchand, R. P. Field observations on swarming and mating in Anopheles gambiae mosquitos in Tanzania. Neth. J. Zool. 34, 367–387 (1984).
Fawaz, E. Y., Allan, S. A., Bernier, U. R., Obenauer, P. J. & Diclaro, J. W. Swarming mechanisms in the yellow fever mosquito: aggregation pheromones are involved in the mating behavior of Aedes aegypti. J. Vector Ecol. 39, 347–354 (2014).
Cabrera, M. & Jaffe, K. An aggregation pheromone modulates lekking behavior in the vector mosquito Aedes aegypti (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 23, 1–10 (2007).
Gjullin, C. M., Whitfield, T. L. & Buckley, J. F. Male pheromones of Culex quinquefasciatus, C. tarsalis and C. pipiens that attract females of these species. Mosq. News 27, 382–387 (1967).
Vas, G. & Vekey, K. Solid-phase microextraction: a powerful sample preparation tool prior to mass spectrometric analysis. J. Mass Spectrom. 39, 233–254 (2004).
Borg-Karlson, A. & Mozuraitis, R. Solid phase microextraction technique used for collecting volatiles released by individual signalling Phyllonorycter sylvella moths. Z. Naturforsch. C 51c, 599–602 (1996).
Millar, J. G. & Sims, J. J. in Methods in Chemical Ecology: Chemical Methods Vol. 1 (eds Millar, J. G. & Haynes, K. F.) 1–37 (Kluwer Academic Publishers, 2000).
Diabaté, A. et al. Mixed swarms of the molecular M and S forms of Anopheles gambiae (Diptera: Culicidae) in sympatric area from Burkina Faso. J. Med. Entomol. 43, 480–483 (2006).
Charlwood, J. D., Thompson, R. & Madsen, H. Observations on the swarming and mating behaviour of Anopheles funestus from southern Mozambique. Malar. J. 2, 2 (2003).
Dabire, K. R. et al. Assortative mating in mixed swarms of the mosquito Anopheles gambiae s.s. M and S molecular forms, in Burkina Faso, West Africa. Med. Vet. Entomol. 27, 298–312 (2013).
Sawadogo, S. P. et al. Differences in timing of mating swarms in sympatric populations of Anopheles coluzzii and Anopheles gambiae s.s. (formerly An. gambiae M and S molecular forms) in Burkina Faso, West Africa. Parasit. Vectors 6, 275 (2013).
Pennetier, C., Warren, B., Dabire, K. R., Russell, I. J. & Gibson, G. “Singing on the wing” as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Curr. Biol. 20, 278–278 (2010).
Somda, N. S. B. et al. Ecology of reproduction of Anopheles arabiensis in an urban area of Bobo-Dioulasso, Burkina Faso (West Africa): monthly swarming and mating frequency and their relation to environmental factors. PLoS ONE 13, e0205966 (2018).
Cator, L. J., Ng’Habi, K. R., Hoy, R. R. & Harrington, L. C. Sizing up a mate: variation in production and response to acoustic signals in Anopheles gambiae. Behav. Ecol. 21, 1033–1039 (2010).
Gibson, G., Warren, B. & Russell, I. J. Humming in tune: sex and species recognition by mosquitoes on the wing. J. Assoc. Res. Otolaryngol. 11, 527–540 (2010).
Carlson, D. A. & Service, M. W. Differentiation between species of the Anopheles gambiae Giles complex (Diptera, Culicidae) by analysis of cuticular hydrocarbons. Ann. Trop. Med. Parasitol. 73, 589–592 (1979).
Carlson, D. A. & Service, M. W. Identification of mosquitos of Anopheles gambiae species complex-A and complex-B by analysis of cuticular components. Science 207, 1089–1091 (1980).
Milligan, P. J. M. et al. A study of the use of gas-chromatography of cuticular hydrocarbons for identifying members of the Anopheles gambiae (Diptera, Culicidae) complex. Bull. Entomol. Res. 83, 613–624 (1993).
Caputo, B. et al. Comparative analysis of epicuticular lipid profiles of sympatric and allopatric field populations of Anopheles gambiae s.s. molecular forms and An. arabiensis from Burkina Faso (West Africa). Insect Biochem. Mol. Biol. 37, 389–398 (2007).
Tripet, F., Dolo, G., Traore, S. & Lanzaro, G. C. The “wingbeat hypothesis” of reproductive isolation between members of the Anopheles gambiae complex (Diptera: Culicidae) does not fly. J. Med. Entomol. 41, 375–384 (2004).
Simoes, P. M. V., Gibson, G. & Russell, I. J. Pre-copula acoustic behaviour of males in the malarial mosquitoes Anopheles coluzzii and Anopheles gambiae s.s. does not contribute to reproductive isolation. J. Exp. Biol. 220, 379–385 (2017).
Pombi, M. et al. Dissecting functional components of reproductive isolation among closely related sympatric species of the Anopheles gambiae complex. Evol. Appl. 10, 1102–1120 (2017).
Lawniczak, M. K. N. et al. Widespread divergence between incipient Anopheles gambiae species revealed by whole genome sequences. Science 330, 512–514 (2010).
Wicker-Thomas, C. Evolution of insect pheromones and their role in reproductive isolation and speciation. Ann. Soc. Entomol. Fr. 47, 55–62 (2011).
Francke, W. & Schulz, S. in Comprehensive Natural Products II: Chemistry and Biology Vol. 4 Chemical Ecology (eds Liu, H. W. & Mander, L.) 153–223 (Elsevier, 2010).
El-Sayed, A. M. The Pherobase: Database of Pheromones and Semiochemicals (Pherobase, 2020); https://www.pherobase.com
Blomquist, G. J. et al. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 40, 699–712 (2010).
Slodowicz, M., Ceriani-Nakamurakare, E., Carmaran, C. & Gonzalez-Audino, P. Sex pheromone component produced by microbial associates of the forest pest Megaplatypus mutatus. Entomol. Exp. Appl. 167, 231–240 (2019).
Xiao, Z. J. & Lu, J. R. Generation of acetoin and its derivatives in foods. J. Agric. Food Chem. 62, 6487–6497 (2014).
de Boer, J. G. et al. Odours of Plasmodium falciparum-infected participants influence mosquito–host interactions. Sci. Rep. 7, 9283 (2017).
Jha, S. K. Characterization of human body odor and identification of aldehydes using chemical sensor. Rev. Anal. Chem. 36, 20160028 (2017).
Tchouassi, D. P. et al. Common host-derived chemicals increase catches of disease-transmitting mosquitoes and can improve early warning systems for Rift Valley fever virus. PLoS Negl. Trop. Dis. 7, e2007 (2013).
Meijerink, J. et al. Identification of olfactory stimulants for Anopheles gambiae from human sweat samples. J. Chem. Ecol. 26, 1367–1382 (2000).
Logan, J. G. et al. Arm-in-cage testing of natural human-derived mosquito repellents. Malar. J. 9, 239 (2010).
Menger, D. J., Van Loon, J. J. A. & Takken, W. Assessing the efficacy of candidate mosquito repellents against the background of an attractive source that mimics a human host. Med. Vet. Entomol. 28, 407–413 (2014).
Nyasembe, V. O. et al. Development and assessment of plant-based synthetic odor baits for surveillance and control of malaria vectors. PLoS ONE 9, e89818 (2014).
Jacob, J. W. et al. Independent and interactive effect of plant- and mammalian-based odors on the response of the malaria vector, Anopheles garnbiae. Acta Trop. 185, 98–106 (2018).
Vanickova, L., Canale, A. & Benelli, G. Sexual chemoecology of mosquitoes (Diptera, Culicidae): current knowledge and implications for vector control programs. Parasitol. Int. 66, 190–195 (2017).
Sawadogo, S. P. et al. Targeting male mosquito swarms to control malaria vector density. PLoS ONE 12, e0173273 (2017).
Emami, S. N., Ranford-Cartwright, L. C. & Ferguson, H. M. The transmission potential of malaria-infected mosquitoes (An. gambiae-Keele, An.arabiensis-Ifakara) is altered by the vertebrate blood type they consume during parasite development. Sci. Rep. 7, 40520 (2017).
Hunt, R. H., Brooke, B. D., Pillay, C., Koekemoer, L. L. & Coetzee, M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Vet. Entomol. 19, 271–275 (2005).
Anderson, J. F. Histopathology of intersexuality in mosquitoes. J. Exp. Zool. 165, 475–484 (1967).
Oliva, C. F., Benedict, M. Q., Lemperiere, G. & Gilles, J. Laboratory selection for an accelerated mosquito sexual development rate. Malar. J. 10, 135 (2011).
Brezolin, A. N. et al. Tools for detecting insect semiochemicals: a review. Anal. Bioanal. Chem. 410, 4091–4108 (2018).
AL-Khshemawee, H., Agarwal, M. & Ren, Y. Evaluation of stable isotope 13C6-glucose on volatile organic compounds in different stages of Mediterranean fruit fly (Medfly) Ceratitis capitate (Diptera: Tephritidae). Entomol. Ornithol. Herpetol. 6, 1–5 (2017).
Hare, D. J. in Methods in Chemical Ecology: Bioassay Methods (eds Millar, J. G. & Haynes, K. F.) 212–270 (Kluwer Academic Publishers, 2000).
Munhenga, G. et al. Mating competitiveness of sterile genetic sexing strain males (GAMA) under laboratory and semi-field conditions: steps towards the use of the sterile insect technique to control the major malaria vector Anopheles arabiensis in South Africa. Parasit. Vectors 9, 122 (2016).
Spitzen, J. et al. A 3D analysis of flight behavior of Anopheles gambiae sensu stricto malaria mosquitoes in response to human odor and heat. PLoS ONE 8, e62995 (2013).
EthoVision XT Base + MAM and Track 3D module-2019 (Noldus, 2019).
Acknowledgements
We thank the Swedish Research Council (Vetenskapsrådet) for funding to S.N.E. (grant no. VR/2017-01229) and (grant no. VR/2017-05543 UFNW) network. We thank Jeanssons Stiftelser (SJ-2018) for the valuable support for setting up the 3D wind-tunnel facility. This project was initially supported by funding awarded to A.-K.B.-K. from the International Atomic Energy Association, grant nos 13733/R1, 13733/R2 and 13733/R3; by Lithuanian state grant “Research into Functions, Responses and Adaptations of Biological Systems and Application Prospects”, Research Program III to R.M.; by L.L.K. and J.W.Z. through the Department of Science and Innovation (DSI)/National Research Foundation (NRF) competitive programme for rated researchers and DSI/NRF South African Research Chairs Initiative Grant (grant no. 171215294399); and Illumina sequencing and Bioinformatics Access Facility to M.H. supported by M.R.F. We thank B. Knols for valuable discussions in the initial stage of the project. We thank L. Ignatowicz and J. Paleovrachas for their support and M. Coetzee for language review and comments.
Author information
Authors and Affiliations
Contributions
A.-K.B.-K., R.M., L.L.K., K.P., M.R.F. and S.N.E. conceived the study. K.P., R.M., M.H., V.S., I.B. and J.S. carried out the laboratory experiments. R.M., L.L.K., J.W.Z. and S.N.E. designed the field experiments. J.W.Z. and S.N.E. collected the field data. R.M. and S.N.E. analysed the data with help from M.H., K.P. and J.S. R.M. and S.N.E. wrote the manuscript. J.S. arranged the figures. J.K.B. and all coauthors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.N.E., R.M. and A.-K.B.-K. are inventors on a patent application (Sw patent application no. ZSE1077999) submitted by the main applicant, S.N.E. (Zacco, Stockholm University), that covers the attraction effects of the aggregation pheromone and the synthetic attractant odour blend. S.N.E. is a cofounder at Molecular Attraction AB. The authors declare no other competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
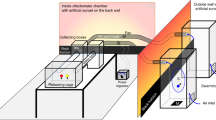
Extended Data Fig. 1 Effect of swarming odours of Anopheles arabiensis (Dongola) and (KGB) as well as Anopheles gambiae (Keele).
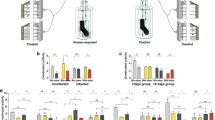
a, The correlation between the number of swarming mosquitoes and amount of odours trapped, males of An. arabiensis (Dongola). b, Amounts of five swarming odours (VOCs) collected during the control, i.e. photophase (C) and transition periods from scotophase to photophase (TSP) and from photophase to scotophase (TPS). The values are taken from the General Linear Mixed Model estimations (GLMM), including the random effect of experimental replication. Significantly different comparisons are indicated by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). In panel b, top of the columns are medians and vertical bars represent standard errors.
Extended Data Fig. 2 Behavioural responses of mosquitoes to control vs control.
Responses of male and female mosquitoes [An. arabiensis (Dongola) and An. gambiae (Keele)] in two-choice olfactometer bioassay were evaluated. The yellow bars show An. arabiensis, and green bars represent An. gambiae panel. It is shown the comparison of Control vs Control: [An. arabiensisi (Dongola) male: χ 21= 0.26, P= 0.601; An. arabiensis (Dongola) female: χ 21= 0.34, P= 0.566; An. gambiae (Keele) male: χ 21= 0.33, P= 0.453; An. gambiae (Keele) female: χ 21= 0.40, P= 0.330].
Extended Data Fig. 3 Behavioural response of Anopheles mosquitoes upon reception of the pheromones.
(1) Stimulated Anopheles males secrete an aggregation pheromone (Chemoemiter) which is a mixture of five volatile compounds including acetoin, sulcatone, octanal, nonanal and decanal. (2) The pheromone mediates formation and sustenance of swarm comprised of flying males. (3) Males respond to the pheromone through antennal sensory organs (Chemoreceiver) with a peak of swarming activity during the photoperiod transition (through the diel and circadian gene regulation). (4) After the male swarm a critical swarm size is initiated achieved, the pheromone enhances female attraction to the swarm and increases mating activity (the section between two arrows). Females respond to the male pheromone (our data), and acoustic signal as an essential signal for coupling (previous literature) with characteristic agitated flight, which serves as attraction stimulus to males in the swarm, inducing males to copulate with females while flying. The graphical illustration is made by Emami group.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, Tables 1–3, gene expression profiling of An. gambiae males, results, discussion, methods and references.
Rights and permissions
About this article
Cite this article
Mozūraitis, R., Hajkazemian, M., Zawada, J.W. et al. Male swarming aggregation pheromones increase female attraction and mating success among multiple African malaria vector mosquito species. Nat Ecol Evol 4, 1395–1401 (2020). https://doi.org/10.1038/s41559-020-1264-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1264-9
This article is cited by
-
Female Aedes aegypti mosquitoes use communal cues to manage population density at breeding sites
Communications Biology (2024)
-
The sex pheromone heptacosane enhances the mating competitiveness of sterile Aedes aegypti males
Parasites & Vectors (2023)
-
Data-driven networking of global transcriptomics and male sexual development in the main malaria vector, Anopheles funestus
Scientific Reports (2023)
-
Combinatorial encoding of odors in the mosquito antennal lobe
Nature Communications (2023)
-
No evidence for long-range male sex pheromones in two malaria mosquitoes
Nature Ecology & Evolution (2022)