Abstract
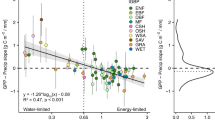
Photosynthesis responds quickly to changes in light, increasing with incoming photosynthetic photon flux density (PPFD) until the leaves become light saturated. This instantaneous response to PPFD, which is widely studied and incorporated into models of photosynthesis, is overlaid on non-instantaneous photosynthetic changes resulting from the acclimation of plants to average PPFD over intermediate timescales of a week to months \(\left( {\overline {{\mathrm{PPFD}}} } \right)\). Such photosynthetic light acclimation is not typically incorporated into models, due to the lack of observational constraints. Here, we use eddy covariance observations from globally distributed and automated sensor networks, along with photosynthesis estimates from nine terrestrial biosphere models (TBMs), to quantify and assess photosynthetic acclimation to light in natural environments. We also use recent theoretical developments to incorporate light acclimation in a TBM. Our results show widespread light acclimation of ecosystem photosynthesis. On average, a 1 μmol m−2 s−1 increase in \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\) (ten-day average PPFD) leads to a 0.031 ± 0.013 μmol C m−2 s−1 increase in the maximum photosynthetic assimilation rate (Amax), with croplands having stronger acclimation rates than grasslands and forests. Our analysis shows that the TBMs examined either neglect or substantially underestimate light acclimation. By updating a TBM to include photosynthetic acclimation, successfully reproducing the \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\)–Amax relationship, we provide a robust method for the incorporation of photosynthetic light acclimation in future models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
This study used openly available eddy covariance measurements provided by the FLUXNET2015 Tier 1 dataset (https://fluxnet.fluxdata.org/data/fluxnet2015-dataset/) and the NACP site-level interim synthesis data downloaded from https://daac.ornl.gov/NACP/. The MODIS fAPAR time series (MOD15A2H) for eddy covariance sites were acquired from https://lpdaac.usgs.gov/tools/appeears.
Code availability
The code to derive the maximum ecosystem photosynthetic rates from eddy covariance measurements is available at https://github.com/bgctw/REddyProc. The code for the optimality model for Vcmax is available at https://github.com/SmithEcophysLab/optimal_vcmax_R. The code for BEPS is available at https://github.com/JChen-UToronto/BEPS_hourly_site_4.02.
References
IPCC Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2013).
Campbell, G. S. & Norman, J. M. An Introduction to Environmental Biophysics (Springer Science & Business Media, 2012).
Lambers, H., Chapin, F. S. & Pons, T. L. Plant Physiological Ecology (Springer New York, 2008).
Ögren, E. & Evans, J. R. Photosynthetic light-response curves. Planta 189, 182–190 (1993).
Fisher, J. B., Huntzinger, D. N., Schwalm, C. R. & Sitch, S. Modeling the terrestrial biosphere. Annu. Rev. Environ. Resour. 39, 91–123 (2014).
Walters, M. B. & Field, C. B. Photosynthetic light acclimation in two rainforest Piper species with different ecological amplitudes. Oecologia 72, 449–456 (1987).
Walters, R. G. & Horton, P. Acclimation of Arabidopsis thaliana to the light environment: changes in composition of the photosynthetic apparatus. Planta 195, 248–256 (1994).
Bailey, S., Walters, R. G., Jansson, S. & Horton, P. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213, 794–801 (2001).
Sims, D. A. & Pearcy, R. W. Photosynthetic characteristics of a tropical forest understory herb, Alocasia macrorrhiza, and a related crop species, Colocasia esculenta grown in contrasting light environments. Oecologia 79, 53–59 (1989).
Poorter, H. et al. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 223, 1073–1105 (2019).
Hikosaka, K. & Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 18, 605–618 (1995).
Warren, C. R. & Adams, M. A. Distribution of N, Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ. 24, 597–609 (2001).
Laisk, A., Nedbal, L. & Govindjee (eds) Photosynthesis in silico (Springer Netherlands, 2009); https://doi.org/10.1007/978-1-4020-9237-4
Baldocchi, D. ‘Breathing’ of the terrestrial biosphere: lessons learned from a global network of carbon dioxide flux measurement systems. Aust. J. Bot. 56, 1–26 (2008).
Baldocchi, D. D. How eddy covariance flux measurements have contributed to our understanding of global change biology. Glob. Change Biol. 26, 242–260 (2020).
Keenan, T. F. et al. Widespread inhibition of daytime ecosystem respiration. Nat. Ecol. Evol. 3, 407–415 (2019).
Prentice, I. C., Dong, N., Gleason, S. M., Maire, V. & Wright, I. J. Balancing the costs of carbon gain and water transport: testing a new theoretical framework for plant functional ecology. Ecol. Lett. 17, 82–91 (2014).
Wang, H. et al. Towards a universal model for carbon dioxide uptake by plants. Nat. Plants 3, 734–741 (2017).
Smith, N. G. et al. Global photosynthetic capacity is optimized to the environment. Ecol. Lett. 22, 506–517 (2019).
Keenan, T. F. et al. Recent pause in the growth rate of atmospheric CO2 due to enhanced terrestrial carbon uptake. Nat. Commun. 7, 13428 (2016).
Maire, V. et al. The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS ONE 7, e38345 (2012).
Bauerle, W. L. et al. Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proc. Natl Acad. Sci. USA 109, 8612–8617 (2012).
He, L. et al. Changes in the shadow: the shifting role of shaded leaves in global carbon and water cycles under climate change. Geophys. Res. Lett. 45, 5052–5061 (2018).
Ögren, E. & Rosenqvist, E. On the significance of photoinhibition of photosynthesis in the field and its generality among species. Photosynth. Res. 33, 63–71 (1992).
Greer, D. H., Berry, J. A. & Björkman, O. Photoinhibition of photosynthesis in intact bean leaves: role of light and temperature, and requirement for chloroplast-protein synthesis during recovery. Planta 168, 253–260 (1986).
Öquist, G., Chow, W. S. & Anderson, J. M. Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 186, 450–460 (1992).
Daniel, E. The temperature dependence of photoinhibition in leaves of Phaseolus vulgaris (L.). Influence of CO2 and O2 concentrations. Plant Sci. 124, 1–8 (1997).
Tyystjärvi, E Photoinhibition of photosystem II. Int. Rev. Cell Mol. Biol. 300, 243–303 (2013).
Katul, G. G., Palmroth, S. & Oren, R. Leaf stomatal responses to vapour pressure deficit under current and CO2-enriched atmosphere explained by the economics of gas exchange. Plant Cell Environ. 32, 968–979 (2009).
Oren, R. et al. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 22, 1515–1526 (1999).
Laing, W. A. Temperature and light response curves for photosynthesis in kiwifruit (Actinidia chinensis) cv. Hayward. N. Zeal. J. Agric. Res. 28, 117–124 (1985).
Leverenz, J. W. The effects of illumination sequence, CO2 concentration, temperature and acclimation on the convexity of the photosynthetic light response curve. Physiol. Plant 74, 332–341 (1988).
Bazzaz, F. A. & Carlson, R. W. Photosynthetic acclimation to variability in the light environment of early and late successional plants. Oecologia 54, 313–316 (1982).
Valladares, F., Martinez-Ferri, E., Balaguer, L., Perez-Corona, E. & Manrique, E. Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: a conservative resource-use strategy? New Phytol. 148, 79–91 (2000).
Poorter, H. & Evans, J. R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116, 26–37 (1998).
Kattge, J., Knorr, W., Raddatz, T. & Wirth, C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Change Biol. 15, 976–991 (2009).
Murchie, E. H. & Horton, P. Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ. 20, 438–448 (1997).
Alter, P., Dreissen, A., Luo, F. L. & Matsubara, S. Acclimatory responses of Arabidopsis to fluctuating light environment: comparison of different sunfleck regimes and accessions. Photosynth. Res. 113, 221–237 (2012).
Slattery, R. A., Walker, B. J., Weber, A. P. M. & Ort, D. R. The impacts of fluctuating light on crop performance. Plant Physiol. 176, 990–1003 (2018).
Murchie, E. H., Hubbart, S., Chen, Y., Peng, S. & Horton, P. Acclimation of rice photosynthesis to irradiance under field conditions. Plant Physiol. 130, 1999–2010 (2002).
Thornton, P. E. & Zimmermann, N. E. An improved canopy integration scheme for a land surface model with prognostic canopy structure. J. Clim. 20, 3902–3923 (2007).
Grant, R. F. et al. Interannual variation in net ecosystem productivity of Canadian forests as affected by regional weather patterns—a FLUXNET-Canada synthesis. Agric. Meteorol. 149, 2022–2039 (2009).
Ryu, Y. et al. Integration of MODIS land and atmosphere products with a coupled-process model to estimate gross primary productivity and evapotranspiration from 1 km to global scales. Glob. Biogeochem. Cycles 25, GB4017 (2011).
Chen, J. M. et al. Vegetation structural change since 1981 significantly enhanced the terrestrial carbon sink. Nat. Commun. 10, 4259 (2019).
Luo, X., Croft, H., Chen, J. M., He, L. & Keenan, T. F. Improved estimates of global terrestrial photosynthesis using information on leaf chlorophyll content. Glob. Change Biol. 25, 2499–2514 (2019).
Pastorello, G. Z. et al. A new data set to keep a sharper eye on land–air exchanges. Eos 98, https://doi.org/10.1029/2017EO071597 (2017).
Lasslop, G. et al. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: critical issues and global evaluation. Glob. Change Biol. 16, 187–208 (2010).
Schaefer, K. et al. A model–data comparison of gross primary productivity: results from the North American Carbon Program site synthesis. J. Geophys. Res. Biogeosci. 117, G03010 (2012).
Ricciuto, D. M. et al. NACP Site: Terrestrial Biosphere Model Output Data in Original Format (ORNL DAAC, 2013); https://doi.org/10.3334/ornldaac/1192
Ricciuto, D. M. et al. NACP Site: Terrestrial Biosphere Model and Aggregated Flux Data in Standard Format (ORNL DAAC, 2013); https://doi.org/10.3334/ornldaac/1183
Gonsamo, A. et al. Improved assessment of gross and net primary productivity of Canada’s landmass. J. Geophys. Res. Biogeosci. 118, 1546–1560 (2013).
Wang, Q. et al. Simulation and scaling of temporal variation in gross primary production for coniferous and deciduous temperate forests. Glob. Change Biol. 10, 37–51 (2004).
Chen, J., Liu, J., Cihlar, J. & Goulden, M. Daily canopy photosynthesis model through temporal and spatial scaling for remote sensing applications. Ecol. Model. 124, 99–119 (1999).
Luo, X. et al. Comparison of big-leaf, two-big-leaf, and two-leaf upscaling schemes for evapotranspiration estimation using coupled carbon–water modeling. J. Geophys. Res. Biogeosci. 123, 207–225 (2018).
Xu, L. & Baldocchi, D. D. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol. 23, 865–877 (2003).
Stocker, B. D. et al. Quantifying soil moisture impacts on light use efficiency across biomes. New Phytol. 218, 1430–1449 (2018).
Fisher, J. B., Whittaker, R. J. & Malhi, Y. ET come home: potential evapotranspiration in geographical ecology. Glob. Ecol. Biogeogr. 20, 1–18 (2011).
Farquhar, G. D., von Caemmerer, S. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Medlyn, B. E. et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 25, 1167–1179 (2002).
Acknowledgements
X.L. and T.F.K. were supported by the NASA Terrestrial Ecology Program IDS Award NNH17AE86I. T.F.K. also acknowledges support by the Director, Office of Science, Office of Biological and Environmental Research of the US Department of Energy under Contract DE-AC02-05CH11231 as part of the RUBISCO SFA. This work used eddy covariance data acquired and shared by the FLUXNET community, including these networks: AmeriFlux, AfriFlux, AsiaFlux, CarboAfrica, CarboEuropeIP, CarboItaly, CarboMont, ChinaFlux, Fluxnet-Canada, GreenGrass, ICOS, KoFlux, LBA, NECC, OzFlux-TERN, TCOS-Siberia and USCCC. The ERA-Interim reanalysis data are provided by ECMWF and processed by LSCE. The FLUXNET eddy covariance data processing and harmonization were carried out by the European Fluxes Database Cluster, AmeriFlux Management Project and Fluxdata project of FLUXNET, with the support of CDIAC and ICOS Ecosystem Thematic Center and the OzFlux, ChinaFlux and AsiaFlux offices. The eddy covariance site used is listed in Supplementary Table 2. We thank NACP for making their site-level synthesis and modelling data publicly available. The TBMs and the eddy covariance sites participating in NACP are acknowledged in Supplementary Tables 3 and 4.
Author information
Authors and Affiliations
Contributions
X.L. and T.F.K. designed the study. X.L. performed the analysis and led the writing. T.F.K. contributed to the writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Photosynthetic light acclimation rate (γA) derived from eddy covariance observations using different time windows (that is 7, 10, 15 and 30 days).
a, the distribution of γA; b, the number of valid bins in which we have at least 20 pairs of Amax and \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\) used to derive γA; c, γA changes with fAPAR; d, the detectability of photosynthetic light acclimation changes with fAPAR; (e) γA changes with Tair; f, the detectability of photosynthetic light acclimation changes with Tair. Note that there are too few samples to plot the changes in γA derived from 30-day window to fAPAR and Tair.
Extended Data Fig. 2 Using ecosystem photosynthesis standardized to PPFD of 2000 µmol m−2 s−1 to study light acclimation.
a, Comparison between Amax and A2000 (that is ecosystem photosynthesis under PPFD of 2000 µmol m−2 s−1) ; b, γA calculated based on the regression of A2000 to \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\); impacts of c, fAPAR and d, daytime air temperature (Tair) on γA calculated based on the regression of A2000 to \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\).
Extended Data Fig. 3 Ecosystem photosynthetic light acclimation explained by light metrics other than light intensity.
The relationships between the maximum photosynthetic rate (Amax) of ecosystems and a, photoperiod (number of daytime hours per day) and b, total amount of photons (mol m−2 day−1) received by vegetation over the same 10-day windows used for \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\).
Extended Data Fig. 4 The fraction of sunlit and shaded leaves under different fAPAR and leaf area index (LAI).
According to Beer’s law, \({\mathrm{fAPAR}} = 1 - {\mathrm{e}}^{ - 0.5{\mathrm{LAI}}}\). The separation of sunlit and shaded leaves is based on a theoretical algorithm23, assuming clumping index is 1 and solar zenith angle is 45°.
Extended Data Fig. 5 The number of pairs of Amax and \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\) for each plant functional type (PFT) to get γA.
The acronyms of PFTs stand for croplands (CRO), deciduous broadleaf forests (DBF), evergreen broadleaf forests (EBF), evergreen needleleaf forests (ENF), mixed forests (MF) and grasslands (GRA).
Extended Data Fig. 6 The rate of photosynthetic light acclimation (γA) for each plant functional type (PFT).
Red indicates the existence of light acclimation and blue means no light acclimation within the bins defined by similar fAPAR and Tair. The black dots indicate where the linear correlation between \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\) and Amax relationship is significant (p < 0.1). The acronyms of PFTs stand for croplands (CRO), deciduous broadleaf forests (DBF), evergreen broadleaf forests (EBF), evergreen needleleaf forests (ENF), mixed forests (MF) and grasslands (GRA).
Extended Data Fig. 7 The number of pairs of maximum photosynthetic rate (Amax) of ecosystems and 10-day average PPFD \(\left( {\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}} \right)\) within each bin.
We only calculated the photosynthetic light acclimation rate (γA) for bins that have more than 20 pairs of Amax and \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\). Each bin is confined by similar fAPAR and Tair, where fAPAR is the fraction of absorbed PPFD, indicating vegetation foliage density and Tair is daytime air temperature.
Extended Data Fig. 8 An example light response curve fitted to eddy covariance measurements.
The y axis is the negative half-hourly net ecosystem exchange (NEE) and the x axis is the half-hourly PPFD. The daytime flux observations in a dynamic window of 2–14 days are used to constrain a light response curve to derive Amax, and every day in the dynamic window is assumed to have the same Amax.
Extended Data Fig. 9 Percentage of the different qualities of the fitted light response curve.
High quality means all parameters in the fitted curve are within a reasonable range, moderate quality means the light response curve is fitted but some of the parameters are out of the reasonable range, and low quality means the light response curve cannot be well constrained using available data in two weeks.
Extended Data Fig. 10 The rate of light acclimation (γA) estimated by 9 terrestrial biosphere models.
Red indicates the existence of light acclimation and blue means no light acclimation within the bins defined by similar fAPAR and Tair. The black dots indicate where the linear correlation between \(\overline {{\mathrm{PPFD}}} _{{\mathrm{10}}}\) and Amax relationship is significant (p < 0.1). The summary of all models is in Supplementary Table 3.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Rights and permissions
About this article
Cite this article
Luo, X., Keenan, T.F. Global evidence for the acclimation of ecosystem photosynthesis to light. Nat Ecol Evol 4, 1351–1357 (2020). https://doi.org/10.1038/s41559-020-1258-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1258-7
This article is cited by
-
Widespread and complex drought effects on vegetation physiology inferred from space
Nature Communications (2023)
-
Atmospheric dryness reduces photosynthesis along a large range of soil water deficits
Nature Communications (2022)
-
Global variation in the fraction of leaf nitrogen allocated to photosynthesis
Nature Communications (2021)
-
Global climate and nutrient controls of photosynthetic capacity
Communications Biology (2021)