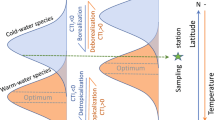
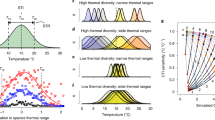
Abstract
Climate change is reshaping global biodiversity as species respond to changing temperatures. However, the net effects of climate-driven species redistribution on local assemblage diversity remain unknown. Here, we relate trends in species richness and abundance from 21,500 terrestrial and marine assemblage time series across temperate regions (23.5–60.0° latitude) to changes in air or sea surface temperature. We find a strong coupling between biodiversity and temperature changes in the marine realm, where species richness mostly increases with warming. However, biodiversity responses are conditional on the baseline climate, such that in initially warmer locations richness increase is more pronounced while abundance declines with warming. In contrast, we do not detect systematic temperature-related richness or abundance trends on land, despite a greater magnitude of warming. As the world is committed to further warming, substantial challenges remain in maintaining local biodiversity amongst the non-uniform inflow and outflow of ‘climate migrants’. Temperature-driven community restructuring is especially evident in the ocean, whereas climatic debt may be accumulating on land.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data can be accessed through either the BioTIME database on Zenodo (https://doi.org/10.5281/zenodo.1211105) or the BioTIME website (http://biotime.st-andrews.ac.uk/).
Code availability
Code to reproduce the analysis is available on GitHub (https://github.com/lauraantao/Temp_Biodiv_Change) and in an online archive at Zenodo59.
References
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003).
Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (2006).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925 (2013).
Bates, A. E. et al. Defining and observing stages of climate-mediated range shifts in marine systems. Glob. Environ. Change 26, 27–38 (2014).
Deutsch, C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (2008).
Cheung, W. W. L., Watson, R. & Pauly, D. Signature of ocean warming in global fisheries catch. Nature 497, 365–368 (2013).
García Molinos, J. et al. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change 6, 83–88 (2016).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830 (2011).
Dillon, M. E., Wang, G. & Huey, R. B. Global metabolic impacts of recent climate warming. Nature 467, 704–706 (2010).
Comte, L. & Olden, J. D. Climatic vulnerability of the world’s freshwater and marine fishes. Nat. Clim. Change 7, 718–722 (2017).
Chen, I.-C., Hill, J. K., Ohlemüller, R., Roy, D. B. & Thomas, C. D. Rapid range shifts of species of climate warming. Science 333, 1024–1026 (2011).
Burrows, M. T. et al. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655 (2011).
Darwin, C. R. On the Origin of Species by Means of Natural Selection (John Murray, 1859).
Lenoir, J. et al. Species better track the shifting isotherms in the oceans than on lands. Preprint at bioRxiv https://doi.org/10.1101/765776 (2019).
Pinsky, M. L., Eikeset, A. M., McCauley, D. J., Payne, J. L. & Sunday, J. M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 569, 108–111 (2019).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690 (2012).
Sunday, J. M. et al. Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot. Ecol. Lett. 18, 944–953 (2015).
Burrows, M. T. et al. Ocean community warming responses explained by thermal affinities and temperature gradients. Nat. Clim. Change 9, 959–963 (2019).
Pinsky, M. L., Worm, B., Fogarty, M. J., Sarmiento, J. L. & Levin, S. A. Marine taxa track local climate velocities. Science 341, 1239–1242 (2013).
Suggitt, A. J. et al. Extinction risk from Climate Change is reduced by microclimatic buffering. Nat. Clim. Change 8, 713–717 (2018).
Supp, S. & Ernest, S. Species-level and community-level responses to disturbance: a cross-community analysis. Ecology 95, 1717–1723 (2014).
Schipper, A. M. et al. Contrasting changes in the abundance and diversity of North American bird assemblages from 1971 to 2010. Glob. Change Biol. 22, 3948–3959 (2016).
Dornelas, M. et al. BioTIME: a database of biodiversity time series for the anthropocene. Glob. Ecol. Biogeogr. 27, 760–786 (2018).
Blowes, S. A. et al. The geography of biodiversity change in marine and terrestrial assemblages. Science 366, 339–345 (2019).
Gotelli, N. J. & Colwell, R. K. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391 (2001).
Jones, P. D. et al. Hemispheric and large-scale land-surface air temperature variations: an extensive revision and an update to 2010. J. Geophys. Res. Atmos. 117, D05127 (2012).
Harris, I., Jones, P. D., Osborn, T. J. & Lister, D. H. Updated high-resolution grids of monthly climatic observations—the CRU TS3.10 Dataset. Int. J. Climatol. 34, 623–642 (2014).
Bowler, D. E. et al. Mapping human pressures on biodiversity across the planet uncovers anthropogenic threat complexes. People Nat. https://doi.org/10.1002/pan3.10071 (2020).
Brown, J., Gillooly, J., Allen, A. & Savage, V. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Edgar, G. J. et al. Abundance and local-scale processes contribute to multi-phyla gradients in global marine diversity. Sci. Adv. 3, e1700419 (2017).
Waldock, C., Dornelas, M. & Bates, A. E. Temperature-driven biodiversity change: disentangling space and time. BioScience 68, 873–884 (2018).
Beaugrand, G., Edwards, M., Raybaud, V., Goberville, E. & Kirby, R. R. Future vulnerability of marine biodiversity compared with contemporary and past changes. Nat. Clim. Change 5, 695–701 (2015).
Pecl, G. T. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214 (2017).
IPCC Climate Change 2014: Impacts, Adaptation, and Vulnerability (eds Field, C. B. et al.) (Cambridge Univ. Press, 2014).
Bates, A. E. et al. Resilience and signatures of tropicalization in protected reef fish communities. Nat. Clim. Change 4, 62–67 (2014).
Bates, A. E., Stuart-smith, R. D., Barrett, N. S. & Edgar, G. J. Biological interactions both facilitate and resist climate-related functional change in temperate reef communities. Proc. R. Soc. B 284, 20170484 (2017).
Devictor, V. et al. Differences in the climatic debts of birds and butterflies at a continental scale. Nat. Clim. Change 2, 121–124 (2012).
Bowler, D. E. et al. Cross-realm assessment of climate change impacts on species’ abundance trends. Nat. Ecol. Evol. 1, 0067 (2017).
Devictor, V., Julliard, R., Couvet, D. & Jiguet, F. Birds are tracking climate warming, but not fast enough. Proc. R. Soc. B 275, 2743–2748 (2008).
Menéndez, R. et al. Species richness changes lag behind climate change. Proc. R. Soc. B 273, 1465–1470 (2006).
Bertrand, R. et al. Ecological constraints increase the climatic debt in forests. Nat. Commun. 7, 12643 (2016).
Meyer, C., Kreft, H., Guralnick, R. & Jetz, W. Global priorities for an effective information basis of biodiversity distributions. Nat. Commun. 6, 8221 (2015).
Dornelas, M. et al. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014).
Williams, J. W., Jackson, S. T. & Kutzbach, J. E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742 (2007).
Nolan, C. et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 361, 920–923 (2018).
Ordonez, A., Williams, J. W. & Svenning, J.-C. Mapping climatic mechanisms likely to favour the emergence of novel communities. Nat. Clim. Change 6, 1104–1109 (2016).
Doak, D. F. & Morris, W. F. Demographic compensation and tipping points in climate-induced range shifts. Nature 467, 959–962 (2010).
Bryndum-Buchholz, A. et al. Twenty-first-century climate change impacts on marine animal biomass and ecosystem structure across ocean basins. Glob. Change Biol. 25, 459–472 (2019).
IPBES Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (eds Díaz, S. et al.) (IPBES Secretariat, 2019).
Şekercioğlu, Ç. H., Primack, R. B. & Wormworth, J. The effects of climate change on tropical birds. Biol. Conserv. 148, 1–18 (2012).
Chao, A. & Jost, L. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93, 2533–2547 (2012).
Wood, S. N. Generalized Additive Models: An Introduction with R (Chapman and Hall/CRC, 2017).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Assis, J. et al. Bio-ORACLE v2.0: extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 27, 277–284 (2018).
Tyberghein, L. et al. Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 21, 272–281 (2012).
Bürkner, P.-C. Advanced Bayesian multilevel modeling with the R package brms. R J. 10, 395–411 (2018).
Bürkner, P.-C. brms: an R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2017).
Antão, L. H. et al. Code relevant for the manuscript ‘Temperature-related biodiversity change across temperate marine and terrestrial systems’. Zenodo https://doi.org/10.5281/zenodo.3708095 (2020).
Sunday, J. et al. Thermal tolerance patterns across latitude and elevation. Phil. Trans. R. Soc. B 374, 20190036 (2019).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
Acknowledgements
We are grateful to all the scientists, data collectors and their funders for making data publicly available. We thank the University of St Andrews Bioinformatics Unit (Wellcome Trust ISSF grant no. 105621/Z/14/Z). L.H.A. acknowledges funding from PBL Netherlands Environmental Assessment Agency as part of the GLOBIO project (www.globio.info), and from the Jane and Aatos Erkko foundation. A.E.B. was supported by the Canada Research Chairs Programme. S.A.B. acknowledges the support of the German Centre of Integrative Biodiversity Research (iDiv) Halle–Jena–Leipzig (funded by the German Research Foundation; no. FZT 118). C.W. was supported by the Natural Environmental Research Council (grant no. 563 NE/L002531/1). M.D. is funded by a Leverhulme Fellowship and by the John Templeton Foundation (grant no. 60501, ‘Putting the Extended Evolutionary Synthesis to the Test’). The BioTIME database was created using funding from the European Research Council (AdG BioTIME (no. 250198) and PoC BioCHANGE (no. 727440)) granted to A.E.M., and we also acknowledge funding from the Leverhulme Centre for Anthropocene Biodiversity. We thank G. Daskalova for valuable input on an early draft of the manuscript and suggestions for the figures. The two icons in the figures are from the Noun Project under CCBY licence: land by A. Skowalsky, and wave by B. Farias.
Author information
Authors and Affiliations
Contributions
M.D. and A.M.S. conceived the idea, and all authors contributed to the design of the project. L.H.A. analysed the data in close consultation with S.A.B., A.E.B., M.D. and A.M.S. L.H.A. wrote the first draft of the manuscript. L.H.A., A.E.B., M.D., A.M.S., S.A.B., C.W., S.R.S. and A.E.M. all contributed to manuscript completion and revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Location of temperate biodiversity time series for each realm.
Dots are coloured according to the temperature change experienced during the period of biodiversity monitoring in those locations (a), and with the corresponding rates of species richness change (b). Credit: b farias, CL (wave image) and Alexander Skowalsky, HU (tree image), The Noun Project (https://thenounproject.com/); world maps produced using the R package tidyverse v.1.2.1 (ref. 61).
Extended Data Fig. 2 Variation among time series regarding temporal sampling.
(a) the starting year (binned into 2-year periods); (b) the number of years sampled; (c) the duration (time period between first and last sample) of the time series; and (d) duration of time series as a function of the number of samples, where colours represent the number of time series for a given combination of duration and number of samples.
Extended Data Fig. 3 Marginal effects of the interaction between temperature change (°C per year) and baseline climate on the biodiversity responses (rate per year).
The coloured fitted lines indicate three long-term annual mean temperature values representing the range across the time series (specifically the mean ± one standard deviation for each realm), and the shaded areas represent the 95% credible interval estimated from the meta-analytical models. For each biodiversity metric, the top row is for marine locations, and the bottom row for terrestrial locations (note the different scales among metrics). Credit: b farias, CL (wave image) and Alexander Skowalsky, HU (tree image), The Noun Project (https://thenounproject.com/).
Extended Data Fig. 4 Comparison of the meta-analytical model estimates using different variables for the baseline climate, as well as latitude.
Dots represent the estimated parameters and whiskers indicate the 95% credible intervals from the Bayesian meta-analysis. Overall, our results were robust to the different temperature variables used: long-term annual mean (Mean_Temp) and maximum temperature (Max_Temp) from the databases WorldClim and Bio-ORACLE, and average air and sea surface temperature in the first year sampled from the HadCRUT4 database (Year1_Temp). Additionally, latitude did not show interacting effects with temperature change.
Extended Data Fig. 5 Sensitivity analysis for each biodiversity metric.
The grey points show the estimated coefficients (and their 95% credible intervals) from 100 meta-analytical models fit to subsets of the marine data, which were randomly subsampled to match the number of locations and latitudinal range of the terrestrial data. Despite the increase in uncertainty due to the smaller data subsets (that is larger credible intervals), comparing the parameter estimates based on the random sub-samples with the parameters estimated using the entire data (blue dots) shows that the marine estimates were not biased due to uneven sampling. Text insets indicate the parameter estimates for the full marine data (blue) and the average across the 100 random sub-samples (grey).
Extended Data Fig. 6 Variation in biodiversity estimated slopes.
Variation in biodiversity estimated slopes as a function of (a) the number of years sampled used to calculate the trends in each time series, where the size of the points represents the duration of the time series (that is Yearend – Yearstart + 1); (b) the duration of the time series; and (c) the start year of each time series, where the size of the points represents the number of years sampled. In all the plots, colours indicate whether estimated slopes were significantly positive (blue), negative (red) or neutral (that is not statistically different to zero; grey).
Extended Data Fig. 7 Variation in biodiversity estimated standard errors.
Variation in biodiversity estimated standard errors as a function of (a) the number of years sampled used to calculate the trends in each time series, where the size of the points represents the duration of the time series (that is Yearend – Yearstart + 1); (b) the duration of the time series; and (c) the start year of each time series, where the size of the points represents the number of years sampled. In all the plots, colours indicate whether estimated slopes were significantly positive (blue), negative (red) or neutral (that is not statistically different to zero; grey).
Extended Data Fig. 8 Density plots of the posterior distributions of the estimated random slopes for the temperature change effect per taxonomic group in the marine realm.
The black vertical line indicates the overall slope estimate for each biodiversity response, with the corresponding 95% credible interval as grey shading; vertical dotted lines indicate zero. The numbers in brackets indicate the number of time series for each taxonomic group (see Supplementary Tables 3–6 for complete model outputs). In two instances, these distributions showed some tendency for deviating from the overall estimated mean. Specifically, “Birds” showed more negative trends for richness change than the average across taxa, and “Multiple taxa” showed more positive trends for abundance change than the average estimate. Yet, both distributions showed extensive overlap with the other taxonomic groups, as well as with the confidence intervals for the overall mean responses. Thus, we refrain from reading too much into these patterns, given the biases in the BioTIME data, and rather focus on the general patterns of change. Credit: b farias, CL (wave image), The Noun Project (https://thenounproject.com/).
Extended Data Fig. 9 Density plots of the posterior distributions of the estimated random slopes for the temperature change effect per taxonomic group in the terrestrial realm.
The black vertical line indicates the overall slope estimate for each biodiversity response, with the corresponding 95% credible interval as grey shading; vertical dotted lines indicate zero. The numbers in brackets indicate the number of time series for each taxonomic group (see Supplementary Tables 3–6 for complete model outputs). Credit: Alexander Skowalsky, HU (tree image), The Noun Project (https://thenounproject.com/).
Supplementary information
Supplementary Information
Supplementary Tables 1–6 and data source references.
Rights and permissions
About this article
Cite this article
Antão, L.H., Bates, A.E., Blowes, S.A. et al. Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat Ecol Evol 4, 927–933 (2020). https://doi.org/10.1038/s41559-020-1185-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1185-7
This article is cited by
-
High temperature delays and low temperature accelerates evolution of a new protein phenotype
Nature Communications (2024)
-
Natural warming differentiates communities and increases diversity in deep-sea Ridge Flank Hydrothermal Systems
Communications Biology (2024)
-
Anthropogenic climate and land-use change drive short- and long-term biodiversity shifts across taxa
Nature Ecology & Evolution (2024)
-
Warming underpins community turnover in temperate freshwater and terrestrial communities
Nature Communications (2024)
-
Sea of opportunities: marine genomics in an era of global environmental change
BMC Genomics (2023)