Abstract
Ectotherms generally shrink under experimental warming, but whether this pattern extends to wild populations is uncertain. We analysed ten million visual survey records, spanning the Australian continent and multiple decades and comprising the most common coastal reef fishes (335 species). We found that temperature indeed drives spatial and temporal changes in fish body size, but not consistently in the negative fashion expected. Around 55% of species were smaller in warmer waters (especially among small-bodied species), while 45% were bigger. The direction of a species’ response to temperature through space was generally consistent with its response to temperature increase through time at any given location, suggesting that spatial trends could help forecast fish responses to long-term warming. However, temporal changes were about ten times faster than spatial trends (~4% versus ~40% body size change per 1 °C change through space and time, respectively). The rapid and variable responses of fish size to warming may herald unexpected impacts on ecosystem restructuring, with potentially greater consequences than if all species were shrinking.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Underwater visual survey datasets are available through the Reef Life Survey site www.reeflifesurvey.com. The final datasets used in this analysis are available through the code depository at https://github.com/astaaudzi/RLSfishSize and as Supplementary datasets linked to this article.
Code availability
All codes used in this analysis are available at https://github.com/astaaudzi/RLSfishSize.
References
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Blanchard, J. L., Heneghan, R. F., Everett, J. D., Trebilco, R. & Richardson, A. J. From bacteria to whales: using functional size spectra to model marine ecosystems. Trends Ecol. Evol. 32, 174–186 (2017).
Fisher, J. A., Frank, K. T. & Leggett, W. C. Breaking Bergmann’s rule: truncation of Northwest Atlantic marine fish body sizes. Ecology 91, 2499–2505 (2010).
Shackell, N. L., Frank, K. T., Fisher, J. A., Petrie, B. & Leggett, W. C. Decline in top predator body size and changing climate alter trophic structure in an oceanic ecosystem. Proc. R. Soc. Lond. B 277, 1353–1360 (2009).
Rouyer, T., Sadykov, A., Ohlberger, J. & Stenseth, N. C. Does increasing mortality change the response of fish populations to environmental fluctuations? Ecol. Lett. 15, 658–665 (2012).
Gardner, J. L., Peters, A., Kearney, M. R., Joseph, L. & Heinsohn, R. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291 (2011).
Cheung, W. W. et al. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 3, 254–258 (2013).
Pauly, D. & Cheung, W. W. L. Sound physiological knowledge and principles in modeling shrinking of fishes under climate change. Glob. Change Biol. 24, e15–e26 (2018).
Lefevre, S., McKenzie, D. J. & Nilsson, G. E. In modelling effects of global warming, invalid assumptions lead to unrealistic projections. Glob. Change Biol. 24, 553–556 (2018).
Morrongiello, J. R., Sweetman, P. C. & Thresher, R. E. Fishing constrains phenotypic responses of marine fish to climate variability. J. Anim. Ecol. 88, 1645–1656 (2019).
Angilletta, M. J. Jr, Steury, T. D. & Sears, M. W. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle. Integr. Comp. Biol. 44, 498–509 (2004).
Bergman, C. Uber die Verhaltnisse der Warmeokonomie der Thiere zu ihrer Grosse. Gottinger Stud. 3, 595–708 (1847).
Audzijonyte, A. et al. Is oxygen limitation in warming waters a valid mechanism to explain decreased body sizes in aquatic ectotherms? Glob. Ecol. Biogeogr. 28, 64–77 (2019).
Forster, J., Hirst, A. G. & Atkinson, D. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc. Natl Acad. Sci. USA 109, 19310–19314 (2012).
Lefevre, S., McKenzie, D. J. & Nilsson, G. E. Models projecting the fate of fish populations under climate change need to be based on valid physiological mechanisms. Glob. Change Biol. 23, 3449–3459 (2017).
Pauly, D. The relationships between gill surface area and growth performance in fish: a generalization of von Bertalanffy’s theory of growth. Meeresforschung 28, 251–282 (1981).
Donelson, J. M., Munday, P. L., McCormick, M. I. & Pitcher, C. R. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32 (2011).
Pecl, G. T. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214 (2017).
Edgar, G. J. & Barrett, N. S. An assessment of population responses of common inshore fishes and invertebrates following declaration of five Australian marine protected areas. Environ. Conserv. 39, 271–281 (2012).
Edgar, G. J. & Stuart-Smith, R. D. Systematic global assessment of reef fish communities by the Reef Life Survey program. Sci. Data 1, 140007 (2014).
Stuart-Smith, R. D., Edgar, G. J., Barrett, N. S., Kininmonth, S. J. & Bates, A. E. Thermal biases and vulnerability to warming in the world’s marine fauna. Nature 528, 88–92 (2015).
Edgar, G. J., Barrett, N. S. & Morton, A. J. Biases associated with the use of underwater visual census techniques to quantify the density and size-structure of fish populations. J. Exp. Mar. Biol. Ecol. 308, 269–290 (2004).
Waldock, C., Stuart‐Smith, R. D., Edgar, G. J., Bird, T. J. & Bates, A. E. The shape of abundance distributions across temperature gradients in reef fishes. Ecol. Lett. 22, 685–696 (2019).
Bennett, S., Wernberg, T., Joy, B. A., De Bettignies, T. & Campbell, A. H. Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 6, 10280 (2015).
Conover, D. O., Duffy, T. A. & Hice, L. A. The covariance between genetic and environmental influences across ecological gradients: reassessing the evolutionary significance of countergradient and cogradient variation. Ann. N. Y. Acad. Sci. 1168, 100–129 (2009).
Audzijonyte, A., Kuparinen, A. & Fulton, E. A. How fast is fisheries‐induced evolution? Quantitative analysis of modelling and empirical studies. Evol. Appl. 6, 585–595 (2013).
Audzijonyte, A. et al. Trends and management implications of human‐influenced life‐history changes in marine ectotherms. Fish Fish. 17, 1005–1028 (2016).
Fritschie, K. J. & Olden, J. D. Disentangling the influences of mean body size and size structure on ecosystem functioning: an example of nutrient recycling by a non‐native crayfish. Ecol. Evol. 6, 159–169 (2016).
AudzijonyteA., KuparinenA., GortonR. & FultonE. A . Ecological consequences of body size decline in harvested fish species: positive feedback loops in trophic interactions amplify human impact. Biol. Lett. 9, 20121103 (2013).
Jørgensen, C. & Fiksen, Ø. Modelling fishing-induced adaptations and consequences for natural mortality. Can. J. Fish. Aquat. Sci. 67, 1086–1097 (2010).
Edgar, G. J. & Stuart-Smith, R. D. Ecological effects of marine protected areas on rocky reef communities—a continental-scale analysis. Mar. Ecol. Prog. Ser. 388, 51–62 (2009).
Reynolds, R. & Banzon, V. NOAA Optimum Interpolation 1/4 Degree Daily Sea Surface Temperature (OISST) Analysis, Version 2 (NOAA National Centers for Environmental Information, 2008); https://go.nature.com/2WtURVO
Stuart-Smith, R. D., Edgar, G. J. & Bates, A. E. Thermal limits to the geographic distributions of shallow-water marine species. Nat. Ecol. Evol. 1, 1846 (2017).
Stan Development Team RStan: the R interface to Stan. R package version 2.19.2 http://mc-stan.org/ (2019).
Acknowledgements
We acknowledge all divers and support personnel who contributed to the data collection and repository, especially A. Cooper, J. Berkhout, E. Oh, J. Stuart-Smith, J. Hulls and E. Clausius. We also thank M. Sumner for help with the SST data, F. Heather for help with figures, J. Berkhout for help with parallel computing, and A. Bates and C. Waldock for thermal distribution information. This research used the NCRIS-enabled Integrated Marine Observing System (IMOS) infrastructure for database support and storage. This study was supported by the ARC Discovery grant DP170104240 (to J.L.B., G.P. and R.D.S.-S.), UTAS visiting scholars fellowship (to A.A. for N.P. visit to IMAS), ARC Future Fellowship to G.P., and the Marine Biodiversity Hub, a collaborative partnership supported through the Australian Government’s National Environmental Science Programme (NESP).
Author information
Authors and Affiliations
Contributions
A.A. designed the study and led the writing. A.A. and S.A.R. designed and conducted the statistical analyses. R.D.S.-S., G.J.E. and N.S.B. led field surveys and data collection. G.P., N.P. and J.L.B. contributed critically to the development of the study. All authors contributed to the manuscript writing and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
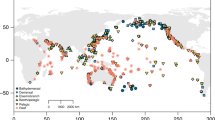
Extended Data Fig. 1 Spatial distribution of Australian fish survey data used, coloured according to the mean annual sea surface temperature.
All sites are grouped onto 0.5 degree grid cells. Colours represent mean annual SST over the entire sampling period in that cell (ranging from 12 °C for yellow to 29 °C for red) and circle size is proportional to the total number of species used for the analyses in the cell (determined by species richness and number of surveys in the cell). Black stars indicate the nine long-term monitoring locations. The Australian coastline shapefile was downloaded from the Australian Natural Resources Data Library website (Commonwealth of Australia).
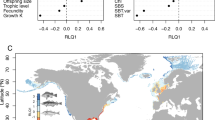
Extended Data Fig. 2 Correlation between species body size – SST slopes and abundance – SST slopes from literature.
Species-specific slopes between body size and mean annual SST through space (β1, on y axis) are compared with the species specific abundance and mean annual SST slopes estimated in Waldock et al. (2019). Data on both slopes was available for 300 species. If abundance was a major driver of average body size, we could expect an overall negative correlation between size-temperature and abundance-temperature slopes, such as mean body size is smaller at sites with higher abundances. Alternatively, larger average body sizes might have positive effects on abundance through e.g. improved recruitment or inter-specific effects. In this case we could expect a positive correlation between size-temperature and abundance-temperature slopes. The correlation between body size slopes and abundances was close to zero (r = 0.09, P = 0.14), suggesting that abundance is unlikely to be a major driver of body sizes and vice versa.
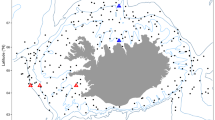
Extended Data Fig. 3 Species-specific body length changes at nine long-term monitored and warming locations.
Location and species specific temporal responses, represented by slopes of body length change in 105 coastal fish species in nine warming and long-term monitored locations. Each dot represents a species, arranged according to the temperature at the centre of their distribution area (temperature midpoint). Blue and red colours indicate species for which 90% of the posterior probability density range for the slope of the annual body length change (on y axis) was above or below zero, respectively. For the map of the nine locations see Extended Data Fig. 1.
Supplementary information
Supplementary Information
Supplementary Tables 2 and 3, and Figs. 1–4.
Supplementary Tables 1 and 4
List of Bayesian estimates of body size–SST slopes through space for each of the 335 individual species used in the analyses (Supplementary Table 1) and Bayesian estimates of annual changes in body lengths (slopes of size versus year) in nine long-term locations (Supplementary Table 4).
Supplementary Data
Filtered and final datasets used in the analyses.
Rights and permissions
About this article
Cite this article
Audzijonyte, A., Richards, S.A., Stuart-Smith, R.D. et al. Fish body sizes change with temperature but not all species shrink with warming. Nat Ecol Evol 4, 809–814 (2020). https://doi.org/10.1038/s41559-020-1171-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1171-0
This article is cited by
-
Life history traits of the Black Sea whiting Merlangius merlangus (Gadidae): compliance with the temperature-size rule
Environmental Biology of Fishes (2024)
-
Cross-basin and cross-taxa patterns of marine community tropicalization and deborealization in warming European seas
Nature Communications (2024)
-
Oxygen availability and body mass modulate ectotherm responses to ocean warming
Nature Communications (2023)
-
Human pressures modulate climate-warming-induced changes in size spectra of stream fish communities
Nature Ecology & Evolution (2023)
-
A diminutive new basilosaurid whale reveals the trajectory of the cetacean life histories during the Eocene
Communications Biology (2023)