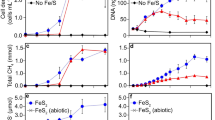
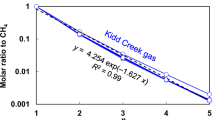
Abstract
Hydrogen gas, H2, is generated by alkaline hydrothermal vents through an ancient geochemical process called serpentinization, in which water reacts with iron-containing minerals deep within the Earth’s crust. H2 is the electron donor for the most ancient and the only energy-releasing route of biological CO2 fixation, the acetyl-CoA pathway. At the origin of metabolism, CO2 fixation by hydrothermal H2 within serpentinizing systems could have preceded and patterned biotic pathways. Here we show that three hydrothermal minerals—greigite (Fe3S4), magnetite (Fe3O4) and awaruite (Ni3Fe)—catalyse the fixation of CO2 with H2 at 100 °C under alkaline aqueous conditions. The product spectrum includes formate (up to 200 mM), acetate (up to 100 µM), pyruvate (up to 10 µM), methanol (up to 100 µM) and methane. The results shed light on both the geochemical origin of microbial metabolism and the nature of abiotic formate and methane synthesis in modern hydrothermal vents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text, Extended Data Figs. 1–10 and the Supplementary Information (Supplementary Methods, Supplementary Tables 1–7, Supplementary Figs. 1–29 and Supplementary Equations).
References
Baross, J. A. & Hoffman, S. E. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph. 15, 327–345 (1985).
McCollom, T. M. Abiotic methane formation during experimental serpentinization of olivine. Proc. Natl Acad. Sci. USA 113, 13965–13970 (2016).
McDermott, J. M., Seewald, J. S., German, C. R. & Sylva, S. P. Pathways for abiotic organic synthesis at submarine hydrothermal fields. Proc. Natl Acad. Sci. USA 112, 7668–7672 (2015).
Ménez, B. et al. Abiotic synthesis of amino acids in the recesses of the oceanic lithosphere. Nature 564, 59–63 (2018).
Klein, F. & Bach, W. Fe-Ni-Co-O-S phase relations in peridotite–seawater interactions. J. Petrol. 50, 37–59 (2009).
Martin, W. F. & Russell, M. J. On the origin of biochemistry at an alkaline hydrothermal vent. Philos. Trans. R. Soc. B 362, 1887–1925 (2007).
Preiner, M. et al. Serpentinization: connecting geochemistry, ancient metabolism and industrial hydrogenation. Life 8, 41 (2018).
Sleep, N. H., Bird, D. K. & Pope, E. C. Serpentinite and the dawn of life. Philos. Trans. R. Soc. B 366, 2857–2869 (2011).
Schrenk, M. O., Brazelton, W. J. & Lang, S. Q. Serpentinization, carbon, and deep life. Rev. Miner. Geochem. 75, 575–606 (2013).
Arndt, N. T. & Nisbet, E. G. Processes on the young Earth and the habitats of early life. Annu. Rev. Earth Planet. Sci. 40, 521–549 (2012).
Fuchs, G. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu. Rev. Microbiol. 65, 631–658 (2011).
Müller, V., Chowdhury, N. P. & Basen, M. Electron bifurcation: a long-hidden energy-coupling mechanism. Annu. Rev. Microbiol. 72, 331–353 (2018).
Ragsdale, S. W. & Pierce, E. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 1784, 1873–1898 (2008).
Sousa, F. L. & Martin, W. F. Biochemical fossils of the ancient transition from geoenergetics to bioenergetics in prokaryotic one carbon compound metabolism. Biochim. Biophys. Acta 1837, 964–981 (2014).
Weiss, M. C. et al. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 1, 16116 (2016).
Huber, C. & Wächtershäuser, G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 276, 245–248 (1997).
He, C., Tian, G., Liu, Z. & Feng, S. A mild hydrothermal route to fix carbon dioxide to simple carboxylic acids. Org. Lett. 12, 649–651 (2010).
Varma, S. J., Muchowska, K. B., Chatelain, P. & Moran, J. Native iron reduces CO2 to intermediates and endproducts of the acetyl-CoA pathway. Nat. Ecol. Evol. 2, 1019–1024 (2018).
Roldan, A. et al. Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions. Chem. Commun. 51, 7501–7504 (2015).
Rajendran, S. & Nasir, S. Hydrothermal altered serpentinized zone and a study of Ni-magnesioferrite–magnetite–awaruite occurrences in Wadi Hibi, Northern Oman Mountain: discrimination through ASTER mapping. Ore Geol. Rev. 62, 211–226 (2014).
Russell, M. J. & Hall, A. J. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. London 154, 377–402 (1997).
Rickard, D. & Luther, G. W. Chemistry of iron sulfides. Chem. Rev. 107, 514–562 (2007).
McCollom, T. M. & Seewald, J. S. Serpentinites, hydrogen, and life. Elements 9, 129–134 (2013).
Hunger, S. & Benning, L. G. Greigite: a true intermediate on the polysulfide pathway to pyrite. Geochem. Trans. 8, 1 (2007).
Findlay, A. J. et al. Iron and sulfide nanoparticle formation and transport in nascent hydrothermal vent plumes. Nat. Commun. 10, 1597 (2019).
Schmitt-Kopplin, P. et al. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl Acad. Sci. USA 107, 2763–2768 (2010).
Dayhoff, M. O. & Eck, R. V. Evolution of the structure of ferredoxin based on surviving relics of primitive amino acid sequences. Science 152, 363–366 (1966).
White, L. M., Bhartia, R., Stucky, G. D., Kanik, I. & Russell, M. J. Mackinawite and greigite in ancient alkaline hydrothermal chimneys: identifying potential key catalysts for emergent life. Earth Planet. Sci. Lett. 430, 105–114 (2015).
Kelley, D. S. et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N. Nature 412, 145–149 (2001).
Kelley, D. S., Baross, J. A. & Delaney, J. R. Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu. Rev. Earth Planet. Sci. 30, 385–491 (2002).
Lang, S. Q., Butterfield, D. A., Schulte, M., Kelley, D. S. & Lilley, M. D. Elevated concentrations of formate, acetate and dissolved organic carbon found at the Lost City hydrothermal field. Geochim. Cosmochim. Acta 74, 941–952 (2010).
Lang, S. Q. et al. Deeply-sourced formate fuels sulfate reducers but not methanogens at Lost City hydrothermal field. Sci. Rep. 8, 755 (2018).
Etiope, G. & Sherwood Lollar, B. Abiotic methane on Earth. Rev. Geophys. 51, 276–299 (2013).
Horita, J. & Berndt, M. E. Abiogenic methane formation and isotopic fractionation under hydrothermal conditions. Sci. Rep. 285, 1055–1057 (1999).
Schuchmann, K. & Müller, V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science 342, 1382–1385 (2013).
Eickenbusch, P. et al. Origin of short-chain organic acids in serpentinite mud volcanoes of the Mariana convergent margin. Front. Microbiol. 10, 1729 (2019).
Etiope, G. & Schoell, M. Abiotic gas: atypical, but not rare. Elements. 10, 291–296 (2014).
McCollom, T. M. & Seewald, J. S. Experimental constraints on the hydrothermal reactivity of organic acids and acid anions: I. Formic acid and formate. Geochim. Cosmochim. Acta 67, 3625–3644 (2003).
McCollom, T. M. & Seewald, J. S. Carbon isotope composition of organic compounds produced by abiotic synthesis under hydrothermal conditions. Earth Planet. Sci. Lett. 243, 74–84 (2006).
McCollom, T. M. & Seewald, J. S. A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine. Geochim. Cosmochim. Acta 65, 3769–3778 (2001).
Menon, S. & Ragsdale, S. W. Unleashing hydrogenase activity in carbon monoxide dehydrogenase/acetyl-CoA synthase and pyruvate:ferredoxin oxidoreductase. Biochemistry 35, 15814–15821 (1996).
Jeoung, J.-H. & Dobbek, H. Carbon dioxide activation at the Ni,Fe-cluster of anaerobic carbon monoxide dehydrogenase. Conserv. Exhib. 318, 1461–1464 (2007).
Dobbek, H., Svetlitchnyi, V., Gremer, L., Huber, R. & Meyer, O. Crystal structure of a carbon monoxide dehydrogenase reveals a [Ni-4Fe-5S] cluster. Science 293, 1281–1285 (2001).
Chabrière, E. et al. Crystal structures of the key anaerobic enzyme pyruvate ferredoxin oxidoreductase free and in complex with pyruvate. Nat. Struct. Biol. 6, 182–190 (1999).
Volbeda, A. et al. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature 373, 580–587 (1995).
Martin, W. F. Carbon-metal bonds: rare and primordial in metabolism. Trends Biochem. Sci. 44, 807–818 (2019).
Buckel, W. & Thauer, R. K. Flavin-based electron bifurcation, ferredoxin, flavodoxin, and anaerobic respiration with protons (Ech) or NAD+(Rnf) as electron acceptors: a historical review. Front. Microbiol. 9, 401 (2018).
Vasiliadou, R., Dimov, N., Szita, N., Jordan, S. & Lane, N. Possible mechanisms of CO2 reduction by H2 via prebiotic vectorial electrochemistry. Interface Focus 9, 20190073 (2018).
Kaufmann, M. On the free energy that drove primordial anabolism. Int. J. Mol. Sci. 10, 1853–1871 (2009).
Patel, B. H., Percivalle, C., Ritson, D. J., Duffy, C. D. & Sutherland, J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015).
Lane, N. & Martin, W. F. The origin of membrane bioenergetics. Cell 151, 1406–1416 (2012).
Jordan, S. F., Nee, E. & Lane, N. Isoprenoids enhance the stability of fatty acid membranes at the emergence of life potentially leading to an early lipid divide. Interface Focus 9, 20100067 (2019).
Jordan, S. F. et al. Promotion of protocell self-assembly from mixed amphiphiles at the origin of life. Nat. Ecol. Evol. 3, 1705–1714 (2019).
Kitadai, N. et al. Metals likely promoted protometabolism in early ocean alkaline hydrothermal systems. Sci. Adv. 5, eaav7848 (2019).
Muchowska, K. B. et al. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 1, 1716–1721 (2017).
Lovley, D. R. & Phillips, E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51, 683–689 (1986).
Igarashi, K., Yamamura, Y. & Kuwabara, T. Natural synthesis of bioactive greigite by solid–gas reactions. Geochim. Cosmochim. Acta 191, 47–57 (2016).
Kato, S., Yumoto, I. & Kamagata, Y. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl. Environ. Microbiol. 81, 67–73 (2015).
Mayumi, D. et al. Carbon dioxide concentration dictates alternative methanogenic pathways in oil reservoirs. Nat. Commun. 4, 1998 (2013).
Deng, X., Chan, C. K. & Tüysüz, H. Spent tea leaf templating of cobalt-based mixed oxide nanocrystals for water oxidation. ACS Appl. Mater. Interfaces 8, 32488–32495 (2016).
Yu, M., Moon, G., Bill, E. & Tüysüz, H. Optimizing Ni−Fe oxide electrocatalysts for oxygen evolution reaction by using hard templating as a toolbox. ACS Appl. Energy Mater. 2, 1199–1209 (2019).
Hanselmann, K. W. Microbial energetics applied to waste repositories. Experientia 47, 645–687 (1991).
Amend, J. P. & Shock, E. L. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic archaea and bacteria. FEMS Microbiol. Rev. 25, 175–243 (2001).
Wang, G., Spivack, A. J. & Hondt, S. D. Gibbs energies of reaction and microbial mutualism in anaerobic deep subseafloor sediments of ODP Site 1226. Geochim. Cosmochim. Acta 74, 3938–3947 (2010).
Wagner, T., Ermler, U. & Shima, S. The methanogenic CO2 reducing-and-fixing enzyme is bifunctional and contains 46 [4Fe-4S] clusters. Science 354, 114–117 (2015).
Mayumi, D. et al. Methane production from coal by a single methanogen. Science 354, 222–225 (2016).
Hoffman, B. M. et al. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 114, 4041–4062 (2014).
Acknowledgements
We thank Y. Dai for setting up gas analysis for the awaruite experiments, A. do Nascimento Vieira for performing parts of the revision experiments, A. Bähr and P. Lim for scientific support and J. C. Xavier for discussions. For funding, J.M., W.F.M. and H.T. thank the VW foundation (no. 96_742). W.F.M. and H.T. thank Deutsche Forschungsgemeinschaft (no. MA-1426/21-1/TU 315/8-1) and W.F.M. thanks the European Research Council (no. ERC 666053). This work is partly supported by IMPRS-RECHARGE and MAXNET Energy consortium of the Max Planck Society. K.I. and Y.K. thank JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas (K.I., no. JP17H05240; Y.K., no. 26106004). K.I. is also supported by Grant-in-Aid for Young Scientists B (no. JP17K15255). J.M. thanks the European Research Council (no. ERC 639170) and ANR LabEX (no. ANR-10-LABX-0026 CSC). This work was also partly supported by Nanotechnology Platform Program (Molecule and Material Synthesis) of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Contributions
W.F.M. wrote the initial draft of the main text and all authors edited the manuscript. W.F.M., H.T., J.M. and M.P. designed the awaruite experiments. M.P. performed the awaruite experiments and assembled the results for the main text and Supplementary Information material. K.B.M. designed and performed the magnetite experiments. S.J.V. performed exploratory experiments with magnetite. Design of the greigite experiments was done by K.I. and Y.K. K.I. performed the experiments. H.T. and M.Y. designed and synthesized the awaruite nanoparticles and performed XRD and transmission electron microscopy measurements for the magnetite and awaruite experiments. M.K.N. performed and interpreted the thermodynamics calculations. K.K., J.M., H.T. and M.P. formulated the H2 reduction mechanism shown in the Supplementary Information.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Overview of the main experiments performed in this study.
Three different iron-containing hydrothermal minerals were tested for their ability to catalyse the reaction between CO2 and H2: greigite (Fe3S4), magnetite (Fe3O4), and awaruite (Ni3Fe).
Extended Data Fig. 2 Background controls for CO2 fixation.
a Control runs for greigite experiments—each run was performed either in the absence of H2 or CO2, or both. Circles show individual measurements. CO2-only and H2-only controls were performed at least in triplicate – values of 0 are not shown by the logarithmic scale. Four types of control experiments under the conditions of greigite experiments were performed with the catalyst: one under 100% CO2 atmosphere, one under 100% H2 atmosphere and two under Ar atmosphere (one with, one without catalyst). The mass spectra of the argon-controls are listed in the supplemental material. The CO2-only experiments show that formate can be formed in small amounts without H2 gas, suggesting that the electrons necessary for CO2 reduction can also come from greigite’s Fe2+ ions, either by forming H2 from water or by a direct reduction of CO2. The latter seems less probable as the step from CO2 to formate is a 2-electron reaction, which electrons Fe2+ cannot provide (see also the proposed mechanism in Extended Data Fig. 10). b Comparison between background and product concentration in awaruite experiments with 1 mmol metal atoms Ni3Fe (16 h at 100 °C, 25 bar, pH > 7). Both CO2 fixation background without Ni3Fe and the background of Ni3Fe itself under an Ar/H2 atmosphere are significantly lower than after H2-dependent CO2 reduction with Ni3Fe. c Comparing background and product concentration in awaruite experiments with 2.5 mmol metal atoms Ni3Fe (16 h at 100 °C, 25 bar, pH > 7). More details on the background contamination in awaruite and magnetite experiments are listed in Supplementary Tables 3–6.
Extended Data Fig. 3 Controls with added Fe0 powder.
a Control for internal Fe0 runs (relevant to magnetite and awaruite experiments). Red: pH < 7; Blue: pH > 7. Circles show individual measurements, all controls were performed in at least triplicate – values of 0 are not shown by the logarithmic scale. As shown in Varma et al.18, a KOH workup after the reaction was necessary prior to analysis. Here we show that KOH can also be added before the reaction (blue bars) in order to liberate the products into the solution. For reactions at lower pH (red bars), KOH was added afterwards. In both cases Fe powder promotes the reduction of CO2 to formate, acetate, pyruvate and methanol. The exact mechanism remains unclear, but it is probable that the Fe powder is being oxidized, leading to the production of H2 from H2O. b Pictures after the reaction at pH 8, 16 h, 100 °C, 25 bar CO2/H2, showing the visual level of oxidation of Fe0, Fe3O4 and Ni3Fe. c XRD of iron powder after a reaction with 0.125 mmol Ni3Fe on top (25 bar CO2, 16 h, pH 8, 100 °C), the results of the CO2 fixation are shown in Extended Data Fig. 6. The XRD spectrum shows that a major part of the iron surface is converted into iron(II) carbonate (siderite, FeCO3), thus confirming the oxidation of the iron powder and the precipitation of Fe2+ ions with carbonate at the same time.
Extended Data Fig. 4 Influence of pH and high-pressure CO experiments for greigite.
a Influence of pH on greigite reactions. Red: pH < 7; Blue: pH > 7. Circles show individual measurements, all measurements performed in at least triplicate. The distribution of carbon fixation products in greigite reactions remains stable with changing pH. b Time course experiment of high-pressure methane and formate production from CO under greigite catalysis (liquid phase, 150 mL) at 60 °C and 100 bar. Using CO gas instead of CO2 and H2 does not explain the amount of methane produced (up to 0.04 mM). The rationale for using CO as a sole reactant stems from previous reports where small organics were obtained in appreciable quantities16. In reactions of CO with greigite and water, no organic products were found other than formate, whose carbon has the same redox state as CO.
Extended Data Fig. 5 Fe0 as external and internal H2 source and ethanol occurrence in awaruite experiments.
a Screen of the quantity of Fe0 as H2 source. As a native metal compound, awaruite could serve as an electron donor and/or a catalyst. To establish the catalytic activity of the nanoparticular awaruite and the ability of Fe0 powder to serve as an H2 delivery system, the concentration of hydrogen gas within the reactor runs was gradually increased. First, 6 neighbouring vials filled with 10 mmol (560 mg) of iron powder (‘external iron’) each provided a small amount of H2 to the atmosphere inside the pressure reactor. By adding 10 mmol iron powder into reaction vials (“internal iron”) and placing the nanoparticular awaruite on top of it, especially formate concentration was pushed towards very high yields (8 mM at pH 5; 100 mM at pH 8, depicted in main Fig. 3b). b Occurrence of ethanol in Ni3Fe reactions. Red: pH < 7; Blue: pH > 7. Circles show individual measurements; all measurements were performed in at least duplicate – values of 0 are not shown by the logarithmic scale. Ethanol was detected in concentrations up to over 0.1 mM. Its concentration fluctuated over the course of experiments, leading to the conclusion that it might be a product of decarboxylation of pyruvate or other side reactions.
Extended Data Fig. 6 Lower amounts of catalyst in magnetite and awaruite experiments.
a Ni3Fe catalysis of CO2 fixation with 0.125 mmol catalyst (0.5 mmol metal atoms). Reactions mixtures with pH < 7 were not treated with KOH after the reaction. b Fe3O4 catalysis of CO2 fixation with 0.167 mmol catalyst (0.5 mmol metal atoms). Reactions mixtures with pH < 7 were treated with KOH after reaction. Using less magnetite and less awaruite still yields a noticeable amount of product. c Ni3Fe catalysis of CO2 fixation at 100–30 °C. Red: pH < 7; Blue: pH > 7.Circles show individual measurements, all measurements were performed in at least triplicate – values of 0 are not shown by the logarithmic scale. Under this thermal gradient (8 h at 100 °C, 8 h at 70 °C, 8 h at 30 °C), even very small amounts (12.5 µmol = 50 µmol of metal atoms) of Ni3Fe suffice to produce notable amounts of formate, acetate and methanol.
Extended Data Fig. 7 Effect of physical contact between Fe0 as internal H2 source and catalyst and effect of Fe0 amount.
a Effect of Fe0 as internal H2 source with magnetite as catalyst. Red: pH < 7; Blue: pH > 7. Circles show individual measurements, all measurements were performed in duplicate – values of 0 are not shown by the logarithmic scale. In the absence of H2 and with Fe0 as reductant, CO2 can be reduced in water in the presence of Fe3O4. Pyruvate accumulates at detectable levels at pH 10 but not at pH 6. The product increase with increasing iron quickly reaches an unexpected maximum, considering the minor differences in product concentration between the two samples with the highest Fe amount. A likely reason is that not the entire bulk of the iron powder reacts/becomes oxidized, but only the surface, thus inhibiting further interaction of the water molecules with the unreacted iron underneath the iron oxide and/or iron carbonate layer formed. b Separating awaruite (0.125 mmol Ni3Fe = 0.5 mmol metal atoms) and internal iron powder. Circles show individual measurements, all measurements in triplicate – values of 0 are not shown by the logarithmic scale. Physical contact between the mineral and native iron is not required for product formation. As shown in Extended Data Fig. 3, placing the catalysts directly on the iron powder in order to “harvest” the nascent hydrogen ascending from the iron powder has the disadvantage that products on the surface of the iron will mix with the ones from awaruite (or magnetite). By tightly packing ca. 10 mg of decontaminated glass wool between iron and awaruite, this effect can be decreased. Although in the initial experiments, no pyruvate could be detected, the other products were formed in appreciable amounts.
Extended Data Fig. 8 Detection of methane by GC-FID for awaruite experiments.
a Gas analysis of 4% (40,000 ppm) methane standard. b CO2/H2 reaction with Ni3Fe (awaruite) as catalyst. These measurements show that CH4 is formed, although only in very small amounts. According to the methane standard measurement, the methane outcome of a typical experiment (16 h, 100 °C, 60:40 CO2/H2 atmosphere, 25 bar) containing 9 mmol of awaruite within one reactor run is roughly 19 ppm (determined by a one-point calibration). Two controls were performed: c one with the reactor pressurized (25 bar) with the 60:40 CO2/H2 gas mixture, and d one after subjecting the CO2/H2 gas mixture to the typical experimental conditions (16 h, 100 °C) within the otherwise empty reactor. Neither control experiment showed traces of methane.
Extended Data Fig. 9 1H-NMR controls for catalysts and reagents and shifts of product peaks in the magnetite and awaruite experiments.
a 1H-NMR material controls for catalysts and reagents used for experiments with NMR product detection. Awaruite and magnetite were tested for surface contamination by treating it with a potassium hydroxide solution to cleave potential contaminants from the surface. Also potassium hydroxide was tested for contaminations. b 1H NMR chemical shifts of product peaks observed and quantified in the magnetite and awaruite experiments. Quantification was achieved using two-point linear regression analysis.
Extended Data Fig. 10 Proposed mechanisms for CO2 reduction with H2 catalysed by hydrothermal minerals.
We propose an ionic mechanism for the catalysed two-electron reduction of CO2 to formate in water for all three minerals a Proposed mechanism for CO2 reduction with H2 catalysed by Fe3O4 (or Fe3S4). An H2 molecule approaches the Fe3O4 (or Fe3S4) surface and reduces Fe3+ to Fe2+. The generated H2+ is unstable and decomposes to H+, assisted by OH– (accounting for increased product formation at pH > 7 in magnetite experiments) – and to a hydrogen atom (H·) which picks up an electron from Fe2+ to become a hydride (H–). Hydride mechanisms were described in previous literature67. CO2 on the other hand physisorbs on the magnetite surface and reacts with the hydride to yield HCOO– which is displaced from the surface by OH–. Observations from experiments not discussed in this publication show, that experiments with minerals only containing ferrous iron (FeO and FeS) give far lower yields of CO2 fixation products. b Proposed mechanism of CO2 reduction catalysed by Ni3Fe. As Ni3Fe only consists of zero-valent metals, H2 can dissociate on the metal surface. Then, the H atoms diffuse into awaruite where they can capture mobile (“free”) electrons from the conduction band of the metal alloy.
Supplementary information
Supplementary Information
Supplementary Tables 1–6, discussion, equations and Figs. 1–29.
Rights and permissions
About this article
Cite this article
Preiner, M., Igarashi, K., Muchowska, K.B. et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat Ecol Evol 4, 534–542 (2020). https://doi.org/10.1038/s41559-020-1125-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1125-6
This article is cited by
-
Heat flows enrich prebiotic building blocks and enhance their reactivity
Nature (2024)
-
To unravel the origin of life, treat findings as pieces of a bigger puzzle
Nature (2024)
-
Generation of long-chain fatty acids by hydrogen-driven bicarbonate reduction in ancient alkaline hydrothermal vents
Communications Earth & Environment (2024)
-
Unique H2-utilizing lithotrophy in serpentinite-hosted systems
The ISME Journal (2023)
-
Ambient temperature CO2 fixation to pyruvate and subsequently to citramalate over iron and nickel nanoparticles
Nature Communications (2023)