Abstract
Steep insect biomass declines ('insectageddon') have been widely reported, despite a lack of continuously collected biomass data from replicated long-term monitoring sites. Such severe declines are not supported by the world’s longest running insect population database: annual moth biomass estimates from British fixed monitoring sites revealed substantial between-year biomass change but no difference in mean biomass between the first (1967–1976) and last decades (2008–2017) of monitoring. High between-year variability and multi-year periodicity in biomass emphasize the need for long-term data to detect trends and identify their causes robustly.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Derived annual biomass data per site analysed in this study are included as Supplementary Dataset 1. Raw data on species-by-night trap catch abundances are retained by RIS and may be obtained by request from https://www.rothamsted.ac.uk/insect-survey.
Code availability
All R scripts, from initial processing of datasets to final analyses, are archived online at https://doi.org/10.5281/zenodo.4442298.
Change history
14 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41559-021-01449-5
References
Hallmann, C. A. et al. PLoS ONE 12, e0185809 (2017).
Lister, B. C. & Garcia, A. Proc. Natl Acad. Sci. USA 115, E10397–E10406 (2018).
Hallmann, C. A. et al. Insect Conserv. Divers. 6, 24451 (2019).
Monbiot, G. Insectageddon: farming is more catastrophic than climate breakdown. The Guardian (20 October 2017).
McGrath, M. Global insect decline may see ‘plague of pests’. BBC Science & Environment (11 February 2019).
Sánchez-Bayo, F. & Wyckhuys, K. A. G. Biol. Conserv. 232, 8–27 (2019).
Komonen, A., Halme, P. & Kotiaho, J. S. ReEco 4, 17–19 (2019).
Thomas, C. D., Jones, T. H. & Hartley, S. E. Glob. Change Biol. 25, 1891–1892 (2019).
Wagner, D. L. Biol. Conserv. 233, 332–333 (2019).
Thomas, J. A. et al. Science 303, 1879–1881 (2004).
Conrad, K. F., Warren, M. S., Fox, R., Parsons, M. S. & Woiwod, I. P. Biol. Conserv. 132, 279–291 (2006).
Fox, R. et al. J. Appl. Ecol. 51, 949–957 (2014).
Wepprich, T., Adrion, J. R., Ries, L., Wiedmann, J. & Haddad, N. M. PLoS ONE 14, e0216270 (2019).
Shortall, C. R. et al. Insect Conserv. Divers. 2, 251–260 (2009).
Kinsella, R. S. et al. Preprint at bioRxiv https://doi.org/10.1101/695635 (2019).
Grubisic, M., van Grunsven, R. H. A., Kyba, C. C. M., Manfrin, A. & Hölker, F. Ann. Appl. Biol. 8, e67798 (2018).
van Langevelde, F. et al. Glob. Change Biol. 24, 925–932 (2018).
Stamp, L. D. Geogr. J. 78, 40–47 (1931).
Fuller, R. M., Groom, G. B., Jones, A. R. & Thomson, A. G. Land Cover Map 1990 (25m raster, GB) (NERC Environmental Information Data Centre, 1993); https://doi.org/10.5285/3d974cbe-743d-41da-a2e1-f28753f13d1e
Morton, D. et al. Countryside Survey Technical Report No. 11/07 (NERC/Centre for Ecology & Hydrology, 2011).
Ditchburn, B., Correia, V., Brewer, A. & Halsall, L. Preliminary Estimates of Change in Woodland Canopy Cover and Woodland Area in Britain Between 2006 and 2015 (National Forest Inventory, 2016).
Sheppard, L. W., Bell, J. R., Harrington, R. & Reuman, D. C. Nat. Clim. Change 6, 610 (2015).
Palmer, G. et al. Phil. Trans. R. Soc. Lond. B 372, 20160144 (2017).
Pescott, O. L. et al. Biol. J. Linn. Soc. Lond. 115, 611–635 (2015).
Andrewartha, H. G. & Birch, L. C. The Distribution and Abundance of Animals (Univ. Chicago Press, 1954).
Taylor, L. R. & Taylor, R. A. Nature 265, 415–421 (1977).
Woiwod, I. P. & Hanski, I. J. Anim. Ecol. 61, 619–629 (1992).
Dirzo, R. et al. Science 345, 401–406 (2014).
Basset, Y. & Lamarre, G. P. A. Science 364, 1230–1231 (2019).
Williams, C. B. Proc. R. Ent. Soc. Lond. A 23, 80–85 (1948).
Storkey, J. et al. Adv. Ecol. Res. 55, 3–42 (2016).
Auffret, A. G. et al. Methods Ecol. Evol. 8, 1453–1457 (2017).
Hollis, D. & McCarthy, M. UKCP09: Met Office Gridded and Regional Land Surface Climate Observation Datasets (Centre for Environmental Data Analysis, 2017).
Gorelick, N. et al. Remote Sens. Environ. 202, 18–27 (2017).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2009).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S (Springer, 2002).
Muggeo, V. M. R. R News 8, 20–25 (2008).
Schwarz, G. Ann. Stat. 6, 461–464 (1978).
Granqvist, E., Hartley, M. & Morris, R. J. Biosystems 110, 60–63 (2012).
Acknowledgements
The RIS, a UK National Capability, is funded by the Biotechnology and Biological Sciences Research Council (BBSRC) under the core capability grant (no. BBS/E/C/000J0200). J.R.B. is also supported by the Smart Crop Protection strategic programme (grant no. BBS/OS/CP/000001) funded through BBSRC’s Industrial Strategy Challenge Fund. We thank P. Verrier and C. Shortall for extracting the data and the RIS team and volunteer network for their unswerving contributions. C.J.M. and C.D.T. were supported by the Natural Environment Research Council (grant no. NE/N015797/1). We thank R. Critchlow and P. Platts for advice on data sources and statistics. We thank C. Harrower, M. Pocock, D. Blumgart and C. Shortall for their help in bringing the data error in the originally published version of this Brief Communication to our attention and investigating its cause.
Author information
Authors and Affiliations
Contributions
C.J.M. and C.D.T. conceived the study. C.J.M. and J.H.W. carried out analyses. J.R.B. provided RIS data and expertise. C.D.T. and C.J.M. drafted the manuscript and all authors commented on it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
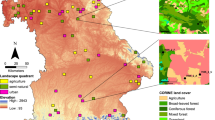
Extended Data Fig. 1 Locations of Rothamsted Insect Survey traps from which data was used in this study.
The dominant land-use class within a circle of 100 m radius surrounding each trap location (from LCM2007; Supplementary Table 6) is indicated by colour (pale orange: arable; mid-blue: grassland; grey: urban; dark green: woodland), and the duration of trapping at each location is indicated by shape (downwards-pointing triangle: eight traps commencing before 1970, with > 6 years of data before the general inflection point; upwards-pointing triangle: 26 traps commencing after 1970, with 6 or fewer years of data before the general inflection point). Three traps in close vicinity to each other, near the Rothamsted Research premises (Harpenden, Hertfordshire, UK), have been manually spaced apart to improve legibility.
Extended Data Fig. 2 Change in biomass of moths over time for the three families that comprise > 90% of total biomass, in each of the four major land-use types.
Zig-zags indicate geometric means of traps operating in each year. A linear regression and a segmented regression was fitted for each combination of family and land use, and lines depict the trend fitted by the better-fitting model (determined using BIC).
Extended Data Fig. 3 Biomass trends at the 34 individual trap sites.
For each site, a linear model and a segmented model were fitted and compared using BIC; the best-fitting model is shown. Where the segmented model was deemed to be best-fitting, the position of the inflection point is shown with 95% confidence intervals. The inflection point from the overall model (1976) is shown as a dotted line, and the number of years of sample data on either side of this point annotated. Traps are ordered left-to-right and then top-to-bottom in order of starting year; note that the capacity to detect inflections is reduced where trapping was initiated from 1980 onwards.
Extended Data Fig. 4 Estimated positions of break-points in biomass trends estimated by all models.
The break-point from each model in Supplementary Table 1 is shown with 95% confidence intervals. Models are grouped by colour, as follows; analysis of full dataset (orange), analyses of specific families (blue), analyses of specific land-use types (red).
Extended Data Fig. 5 Change in biomass of moths over time is not a result of changing land use.
Land-use type was determined for each site using land cover datasets published in 1931, 1990 and 2007. Comparing between a 1990 and 2007 and b 1931 and 2007, each site was classified according to whether it had the same land-use type in both years or had changed in the interim (possibly due to methodological artefacts, rather than true land-use change). Here, data are shown only from sites which were consistently classified to the same land-use type in both years. Biomass at individual traps sites is shown in grey, and geometric mean as a black zig-zag. Black line depicts the trend fitted by the best-fitting model from a segmented regression and a linear regression.
Extended Data Fig. 6 Posterior distribution function (PDF) for Bayesian Spectrum Analysis of annual change in biomass.
Bayesian Spectrum Analysis was conducted on mean values for proportional biomass change across all traps in each year, with a prior probability distribution for frequency of cycles of 2–20 years. The density of the PDF at each value of years per cycle indicates the relative likelihood that the data represents a cycle with that frequency. The results indicate a high overall chance ( > 97.5%) of there being a pattern to biomass change that exceeds two years but low certainty of its exact period. This is consistent with the hypothesis that there are perturbations and ‘return times’ rather than ‘true’ periodicity in the data.
Extended Data Fig. 7 Relationships between biomass change, estimated by linear regression of annual data and by the difference between two samples from the same site, and duration of data subsets.
All possible subsets of our full dataset (of at least five years duration) were taken, as well as all possible subsets of the data for each separate trap (of at least five years length, and consisting of data from at least five years to account for years when some traps were unrecorded). For each data subset, we calculated change per year in biomass in two ways: (i) as the percentage change per year predicted by a linear regression through all years of data, and (ii) as the percentage change per year between biomass in the first and last years of the data subset (to mimic studies where a particular location might only be sampled twice, with an interval of five or more years). Panels a, b show the results for the linear regression approach: a across all traps (each point represents the average across all traps for a given duration/start date combination), and b for each trap. Panels c, d show results for the first-last year analyses: c across all traps, and d for each trap. Panels e-f show the correspondence between these two types of analysis (that is, regression versus first-last date) when applied to the same duration/start date combination. The two approaches commonly agree but not always. Blue points in e-f indicate data subsets where both approaches to estimating biomass change were in agreement about the direction of change (increase or decline), and orange points highlight subsets where the methods produced different outcomes. In panels b, d, and f, points are plotted with 95% transparency to visualize density of overplotted points.
Extended Data Fig. 8 Effect of starting date (baseline) on estimated rates of biomass change, for 20-year periods.
All possible 20-year long subsets of our full dataset were taken (black lines), as well as all possible 20-year long subsets of the data for each individual trap (grey lines). We calculated change in biomass per year in two ways (regression over all years versus difference between first-last years), as for Extended Data Fig 7. Panels show a regressed percentage change per year, and b percentage change per year between first and last years, against onset of the data subset. Twenty-year time sequences that originate before the late 1970s (that is, before the 1976 peak) suggest increases in biomass over time, whereas sequences originating later than this typically indicate declines in biomass. The two-sample estimates (black zig-zag line in b) are much noisier than those based on continuous sampling (black curve-like line in a).
Extended Data Fig. 9 Distribution of inoperative days through the year.
Panel a shows the number of all trap/year combinations which were inoperative on each Julian day of the year (median: 1.56 % of trap-years inoperative), and reveals that inoperative days were distributed evenly across the entire year (other than a slight increase around Christmas/New Year, when few moths are active). Panel b shows the frequency of inoperative days per trap/year combination, expressed as a percentage of all days in the year, with the median value (0.55 %) shown as a dashed line. Bins are 1 % wide.
Supplementary information
Supplementary Information
Supplementary Tables 1–6.
41559_2019_1028_MOESM3_ESM.xlsx
Supplementary Dataset 1 Annual estimated biomass of moths at each of the 34 traps. For each trap, in each year, the total estimated biomass of moths sampled (adjusted for the number of inoperative days) is provided, along with the total estimated biomass of each of the three families that collectively comprised >93 % of all moth biomass (Erebidae, Geometridae and Noctuidae).
Rights and permissions
About this article
Cite this article
Macgregor, C.J., Williams, J.H., Bell, J.R. et al. Moth biomass has fluctuated over 50 years in Britain but lacks a clear trend. Nat Ecol Evol 3, 1645–1649 (2019). https://doi.org/10.1038/s41559-019-1028-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-019-1028-6
This article is cited by
-
Weather explains the decline and rise of insect biomass over 34 years
Nature (2024)
-
Urbanization related changes in lepidopteran community
Urban Ecosystems (2024)
-
Insect decline in forests depends on species’ traits and may be mitigated by management
Communications Biology (2023)
-
Spatial sorting promotes rapid (mal)adaptation in the red-shouldered soapberry bug after hurricane-driven local extinctions
Nature Ecology & Evolution (2023)
-
Positive shifts in species richness and abundance of moths over five decades coincide with community-wide phenotypic trait homogenisation
Journal of Insect Conservation (2023)