Abstract
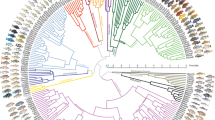
Adaptive radiation illustrates links between ecological opportunity, natural selection and the generation of biodiversity. Central to adaptive radiation is the association between a diversifying lineage and the evolution of phenotypic variation that facilitates the use of new environments or resources. However, is not clear whether adaptive evolution or historical contingency is more important for the origin of key phenotypic traits in adaptive radiation. Here we use targeted sequencing of >250,000 loci across 46 species to examine hypotheses concerning the origin and diversification of key traits in the adaptive radiation of Antarctic notothenioid fishes. Contrary to expectations of adaptive evolution, we show that notothenioids experienced a punctuated burst of genomic diversification and evolved key skeletal modifications before the onset of polar conditions in the Southern Ocean. We show that diversifying selection in pathways associated with human skeletal dysplasias facilitates ecologically important variation in buoyancy among Antarctic notothenioid species, and demonstrate the sufficiency of altered trip11, col1a2 and col1a1a function in zebrafish (Danio rerio) to phenocopy skeletal reduction in Antarctic notothenioids. Rather than adaptation being driven by the cooling of the Antarctic, our results highlight the role of historical contingency in shaping the adaptive radiation of notothenioids. Understanding the historical and environmental context for the origin of key traits in adaptive radiations extends beyond reconstructing events that result in evolutionary innovation, as it also provides a context in forecasting the effects of climate change on the stability and evolvability of natural populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The sequencing data have been deposited in the NCBI database as Bioproject PRJNA531677. Assembled contig data have been deposited in the Zenodo repository (10.5281/zenodo.2628936).
Change history
10 March 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Schluter, D. The Ecology of Adaptive Radiation (Oxford Univ. Press, 2000).
Chan, Y. F. et al. Adaptive evolution of pelvic reduction of a Pitx1 enhancer. Science 327, 302–306 (2010).
Santos, M. E. et al. The evolution of cichlid fish egg-spots is linked with a cis-regulatory change. Nat. Commun. 5, 5149 (2014).
Rabosky, D. L. Phylogenetic tests for evolutionary innovation: the problematic link between key innovations and exceptional diversification. Phil. Trans. R. Soc. B 372, 20160417 (2017).
Stroud, J. T. & Losos, J. B. Ecological opportunity and adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 47, 507–532 (2016).
Losos, J. B. Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles (Univ. of California Press, 2009).
Gould, S. J. Wonderful Life: The Burgess Shale and the Nature of History (W. W. Norton & Co., 1989).
Gould, S. J. The Structure of Evolutionary Theory (Harvard Univ. Press, 2002).
Jablonski, D. Approaches to macroevolution: 1. General concepts and origin of variation. Evol. Biol. 44, 427–450 (2017).
Near, T. J. et al. Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proc. Natl Acad. Sci. USA 109, 3434–3439 (2012).
Dornburg, A., Federman, S., Lamb, A. D., Jones, C. D. & Near, T. J. Cradles and museums of Antarctic teleost biodiversity. Nat. Ecol. Evol. 1, 1379–1384 (2017).
Eastman, J. T. Antarctic Fish Biology: Evolution in a Unique Environment (Academic Press, Inc., 1993).
DeVries, A. L. & Eastman, J. T. Lipid sacs as a buoyancy adaptation in an Antarctic fish. Nature 271, 352–353 (1978).
Eastman, J. T., Witmer, L. M., Ridgely, R. C. & Kuhn, K. L. Divergence in skeletal mass and bone morphology in Antarctic notothenioid fishes. J. Morphol. 275, 841–861 (2014).
Daane, J. M., Rohner, N., Konstantinidis, P., Djuranovic, S. & Harris, M. P. Parallelism and epistasis in skeletal evolution identified through use of phylogenomic mapping strategies. Mol. Biol. Evol. 33, 162–173 (2016).
Near, T. J., Parker, S. K. & Detrich, H. W. A genomic fossil reveals key steps in hemoglobin loss by the Antarctic icefishes. Mol. Biol. Evol. 23, 2008–2016 (2006).
Brawand, D. et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature 513, 375–381 (2014).
Rabosky, D. L. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS ONE 9, e89543 (2014).
Gistelinck, C. et al. Zebrafish type I collagen mutants faithfully recapitulate human type I collagenopathies. Proc. Natl Acad. Sci. USA 115, E8037–E8046 (2018).
Van Dijk, F. S. & Sillence, D. O. Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am. J. Med. Genet. A 164, 1470–1481 (2014).
Albertson, R. C. et al. Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evol. Biol. 10, 4 (2010).
Witkos, T. M. & Lowe, M. The golgin family of coiled-coil tethering proteins. Front. Cell Dev. Biol. 3, 86 (2016).
Smits, P. et al. Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N. Engl. J. Med. 362, 206–216 (2010).
Eastman, J. T. & McCune, A. R. Fishes on the Antarctic continental shelf: evolution of a marine species flock? J. Fish Biol. 57, 84–102 (2000).
Chen, L., DeVries, A. & Cheng, C. Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc. Natl Acad. Sci. USA 94, 3811–3816 (1997).
Chen, Z. et al. Transcriptomic and genomic evolution under constant cold in Antarctic notothenioid fish. Proc. Natl Acad. Sci. USA 105, 12944–12949 (2008).
Chown, S. L. et al. The changing form of Antarctic biodiversity. Nature 522, 431–438 (2015).
Chown, S. L. et al. Antarctica and the strategic plan for biodiversity. PLoS Biol. 15, e2001656 (2017).
Bilyk, K. T., Vargas-Chacoff, L. & Cheng, C. H. C. Evolution in chronic cold: varied loss of cellular response to heat in Antarctic notothenioid fish. BMC Evol. Biol. 18, 143 (2018).
Shin, S. C. et al. The g enome sequence of the Antarctic bullhead notothen reveals evolutionary adaptations to a cold environment. Genome Biol. 15, 468 (2014).
Tine, M. et al. European sea bass genome and its variation provide insights into adaptation to euryhalinity and speciation. Nat. Commun. 5, 5770 (2014).
Herrero, J. et al. Ensembl comparative genomics resources. Database 2016, bav096 (2016).
Kozomara, A. & Griffiths-Jones, S. MiRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 39, D152–D157 (2011).
Dimitrieva, S. & Bucher, P. UCNEbase - a database of ultraconserved non-coding elements and genomic regulatory blocks. Nucleic Acids Res. 41, 101–109 (2013).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Altschul, S., Gish, W. & Miller, W. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Huang, X. CAP3: a DNA sequence assembly program. Genome Res. 9, 868–877 (1999).
Sedlazeck, F. J., Rescheneder, P. & Von Haeseler, A. NextGenMap: fast and accurate read mapping in highly polymorphic genomes. Bioinformatics 29, 2790–2791 (2013).
Katoh, K., Kuma, K., Toh, H. & Miyata, T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33, 511–518 (2005).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Robinson, J. T. et al. Integrative genome viewer. Nat. Biotechnol. 29, 24–26 (2011).
Henke, K. et al. Genetic screen for post-embryonic development in the zebrafish (Danio rerio): dominant mutations affecting adult form. Genetics 207, 609–623 (2017).
Nguyen, L. T., Schmidt, H. A., Von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Chen, K., Durand, D. & Farach-Colton, M. NOTUNG: a program for dating gene duplications and optimizing gene family trees. J. Comput. Biol. 7, 429–447 (2000).
Ranwez, V., Harispe, S., Delsuc, F. & Douzery, E. J. P. MACSE: multiple alignment of coding SEquences accounting for frameshifts and stop codons. PLoS ONE 6, e22594 (2011).
Sela, I., Ashkenazy, H., Katoh, K. & Pupko, T. GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 43, W7–W14 (2015).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., Von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Hoang, D. T., Chernomor, O., Von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Kubatko, L. S. & Degnan, J. H. Inconsistency of phylogenetic estimates from concatenated data under coalescence. Syst. Biol. 56, 17–24 (2007).
Roch, S. & Steel, M. Likelihood-based tree reconstruction on a concatenation of aligned sequence data sets can be statistically inconsistent. Theor. Popul. Biol. 100, 56–62 (2015).
Zhang, C., Rabiee, M., Sayyari, E. & Mirarab, S. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19, 15–30 (2018).
Sayyari, E. & Mirarab, S. Fast coalescent-based computation of local branch support from quartet frequencies. Mol. Biol. Evol. 33, 1654–1668 (2016).
Rabosky, D. L. et al. BAMMtools: an R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods Ecol. Evol. 5, 701–707 (2014).
Bouckaert, R. et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 10, e1003537 (2014).
Bouckaert, R. R. & Drummond, A. J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 17, 42 (2017).
Drummond, A. J. & Suchard, M. A. Bayesian random local clocks, or one rate to rule them all. BMC Biol. 8, 114 (2010).
Near, T. J. et al. Identification of the notothenioid sister lineage illuminates the biogeographic history of an Antarctic adaptive radiation. BMC Evol. Biol. 15, 109 (2015).
Smith, M. D. et al. Less is more: an adaptive branch-site random effects model for efficient detection of episodic diversifying selection. Mol. Biol. Evol. 32, 1342–1353 (2015).
Kosakovsky Pond, S. L., Frost, S. D. W. & Muse, S. V. HyPhy: hypothesis testing using phylogenies. Bioinformatics 21, 676–679 (2005).
Pollard, K. S., Hubisz, M. J., Rosenbloom, K. R. & Siepel, A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 20, 110–121 (2010).
Hubisz, M. J., Pollard, K. S. & Siepel, A. PHAST and RPHAST: phylogenetic analysis with space/time models. Brief. Bioinforma. 12, 41–51 (2011).
Kinsella, R. J. et al. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database 2011, bar030 (2011).
Köhler, S. et al. The human phenotype ontology in 2017. Nucleic Acids Res. 45, D865–D876 (2017).
Daub, J. T., Moretti, S., Davydov, I. I. & Excoffier, L. Detection of pathways affected by positive selection in primate lineages ancestral to humans. Mol. Biol. Evol. 34, 1391–1402 (2017).
Nüsslein-Volhard, C. & Dahm, R. Zebrafish: A Practical Approach (Oxford Univ. Press, 2002).
Acknowledgements
The authors thank E. Snay and L. Oberg in the Department of Nuclear Medicine and Molecular Imaging at Boston Children’s Hospital for assistance in computed tomography of adult specimens. This work was supported in part by American Heart Association Postdoctoral Fellowship (No. 17POST33660801) to J.M.D., the National Institutes of Health (NIH) (No. U01DE024434), the John Simon Guggenheim Fellowship and William F. Milton Fund awarded to M.P.H., and the National Science Foundation (NSF) (No. PLR-1444167 to H.W.D. and No. IOS-1755242 to A.D.), the Bingham Oceanographic Fund from the Peabody Museum of Natural History, and Yale University, as well as the Children’s Orthopaedic Surgery Foundation at Boston Children’s Hospital. This is contribution No. 389 from the Marine Science Center at Northeastern University.
Author information
Authors and Affiliations
Contributions
J.M.D., H.W.D. and M.P.H. conceived and designed the study. J.M.D., P.S. and M.B.H. performed the experiments. J.M.D., A.D., T.J.N., D.J.M., H.W.D. and M.P.H. analysed the data. J.M.D., A.D. and T.J.N. wrote the first drafts of the manuscript. All authors contributed to the writing of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13 and Supplementary Tables 1–11
Rights and permissions
About this article
Cite this article
Daane, J.M., Dornburg, A., Smits, P. et al. Historical contingency shapes adaptive radiation in Antarctic fishes. Nat Ecol Evol 3, 1102–1109 (2019). https://doi.org/10.1038/s41559-019-0914-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-019-0914-2
This article is cited by
-
Genomics of cold adaptations in the Antarctic notothenioid fish radiation
Nature Communications (2023)
-
A latitudinal gradient of deep-sea invasions for marine fishes
Nature Communications (2023)
-
Placing human gene families into their evolutionary context
Human Genomics (2022)
-
Population genomics of an icefish reveals mechanisms of glacier-driven adaptive radiation in Antarctic notothenioids
BMC Biology (2022)
-
Prolonged morphological expansion of spiny-rayed fishes following the end-Cretaceous
Nature Ecology & Evolution (2022)