Abstract
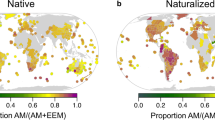
Island biogeography has traditionally focused primarily on abiotic drivers of colonization, extinction and speciation. However, establishment on islands could also be limited by biotic drivers, such as the absence of symbionts. Most plants, for example, form symbioses with mycorrhizal fungi, whose limited dispersal to islands could act as a colonization filter for plants. We tested this hypothesis using global-scale analyses of ~1.4 million plant occurrences, including ~200,000 plant species across ~1,100 regions. We find evidence for a mycorrhizal filter (that is, the filtering out of mycorrhizal plants on islands), with mycorrhizal associations less common among native island plants than native mainland plants. Furthermore, the proportion of native mycorrhizal plants in island floras decreased with isolation, possibly as a consequence of a decline in symbiont establishment. We also show that mycorrhizal plants contribute disproportionately to the classic latitudinal gradient of plant species diversity, with the proportion of mycorrhizal plants being highest near the equator and decreasing towards the poles. Anthropogenic pressure and land use alter these plant biogeographical patterns. Naturalized floras show a greater proportion of mycorrhizal plant species on islands than in mainland regions, as expected from the anthropogenic co-introduction of plants with their symbionts to islands and anthropogenic disturbance of symbionts in mainland regions. We identify the mycorrhizal association as an overlooked driver of global plant biogeographical patterns with implications for contemporary island biogeography and our understanding of plant invasions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data used in this manuscript are available on request (GIFT and GloNAF databases). The GloNAF database has been published and available for use at https://esajournals.onlinelibrary.wiley.com/doi/abs/10.1002/ecy.2542. The code for plotting data from GIFT is available at https://github.com/BioGeoMacro/GIFT-export. All family mycorrhizal proportion data are available in Supplementary Table 2, and data summarizing the proportions of mycorrhizal and non-mycorrhizal species per region are supplied in Supplementary Table 4.
References
MacArthur, R. H. & Wilson, E. The Theory of Island Biogeography (Princeton Univ. Press, Princeton, 1967).
Losos, J. B. & Schluter, D. Analysis of an evolutionary species–area relationship. Nature 408, 847–850 (2000).
Kisel, Y. & Barraclough, T. G. Speciation has a spatial scale that depends on levels of gene flow. Am. Nat. 175, 316–334 (2010).
Borregaard, M. K. et al. Oceanic island biogeography through the lens of the general dynamic model: assessment and prospect. Biol. Rev. 92, 830–853 (2017).
Kreft, H., Jetz, W., Mutke, J., Kier, G. & Barthlott, W. Global diversity of island floras from a macroecological perspective. Ecol. Lett. 11, 116–127 (2008).
Weigelt, P., Steinbauer, M. J., Cabral, J. S. & Kreft, H. Late Quaternary climate change shapes island biodiversity. Nature 532, 99–102 (2016).
Whittaker, R. J., Triantis, K. A. & Ladle, R. J. A general dynamic theory of oceanic island biogeography. J. Biogeogr. 35, 977–994 (2008).
Losos, J. B. & Ricklefs, R. E. The theory of Island Biogeography Revisited (Princeton Univ. Press, Princeton, 2009).
Onstein, R. E. et al. Frugivory-related traits promote speciation of tropical palms. Nat. Ecol. Evol. 1, 1903–1911 (2017).
Fukami, T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst. 46, 1–23 (2015).
Bever, J. D., Westover, K. M. & Antonovics, J. Incorporating the soil community into plant population dynamics: the utility of the feedback approach. Ecology 85, 561–573 (1997).
Van der Heijden, M. G., Bardgett, R. D. & van Straalen, N. M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310 (2008).
Delavaux, C. S., Smith‐Ramesh, L. M. & Kuebbing, S. E. Beyond nutrients: a meta‐analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils.Ecology 98, 2111–2119 (2017).
Redecker, D., Kodner, R. & Graham, L. E. Glomalean fungi from the Ordovician. Science 289, 1920–1921 (2000).
Maherali, H. et al. Mutualism persistence and abandonment during the evolution of the mycorrhizal symbiosis. Am. Nat. 188, E113–E125 (2016).
Smith, S. E. & Read, D. J. Mycorrhizal Symbiosis (Academic Press, Oxford, UK, 2008).
Davison, J. et al. Hierarchical assembly rules in arbuscular mycorrhizal (AM) fungal communities. Soil Biol. Biochem. 97, 63–70 (2016).
Dickie, I. A. et al. The emerging science of linked plant–fungal invasions. New Phytol. 215, 1314–1332 (2017).
Miller, R. M., Smith, C. I., Jastrow, J. D. & Bever, J. D. Mycorrhizal status of the genus Carex (Cyperaceae). Am. J. Bot. 86, 547–553 (1999).
Brundrett, M. C. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320, 37–77 (2009).
Tedersoo, L. Biogeography of Mycorrhizal Symbiosis Vol. 230 (Springer, Cham, Switzerland, 2017).
Brundrett, M. C. & Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity.New Phytol. 220, 1108–1115 (2018).
Lambers, H., Raven, J. A., Shaver, G. R. & Smith, S. E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 23, 95–103 (2008).
Jiang, S. et al. Dynamics of arbuscular mycorrhizal fungal community structure and functioning along a nitrogen enrichment gradient in an alpine meadow ecosystem.New Phytol. 220, 1222–1235 (2018).
Abbott, K. C. et al. Spatial heterogeneity in soil microbes alters outcomes of plant competition. PLoS ONE 10, e0125788 (2015).
Steidinger, B. S. & Bever, J. D. The coexistence of hosts with different abilities to discriminate against cheater partners: an evolutionary game-theory approach. Am. Nat. 183, 762–770 (2014).
Davison, J. et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349, 970–973 (2015).
Tedersoo, L. et al. Global diversity and geography of soil fungi. Science 346, 1256688 (2014).
Peay, K. G., Schubert, M. G., Nguyen, N. H. & Bruns, T. D. Measuring ectomycorrhizal fungal dispersal: macroecological patterns driven by microscopic propagules. Mol. Ecol. 21, 4122–4136 (2012).
Peay, K. G. Timing of mutualist arrival has a greater effect on Pinus muricata seedling growth than interspecific competition. J. Ecol. 106, 514–523 (2018).
Koziol, L. et al. The plant microbiome and native plant restoration: the example of native mycorrhizal fungi.BioScience 68, 996–1006 (2018).
Richardson, D. M., Allsopp, N., D’Antonio, C. M., Milton, S. J. & Rejmánek, M. Plant invasions—the role of mutualisms. Biol. Rev. 75, 65–93 (1999).
Callaway, R. M., Thelen, G. C., Rodriguez, A. & Holben, W. E. Soil biota and exotic plant invasion. Nature 427, 731–733 (2004).
Oehl, F. et al. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 69, 2816–2824 (2003).
Pringle, A. et al. Mycorrhizal symbioses and plant invasions. Annu. Rev. Ecol. Evol. Syst. 40, 699–715 (2009).
Bunn, R. A., Ramsey, P. W. & Lekberg, Y. Do native and invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta-analysis. J. Ecol. 103, 1547–1556 (2015).
Reinhart, K. O., Lekberg, Y., Klironomos, J. & Maherali, H. Does responsiveness to arbuscular mycorrhizal fungi depend on plant invasive status?. Ecol. Evol. 7, 6482–6492 (2017).
Bueno, C. G. et al. Plant mycorrhizal status, but not type, shifts with latitude and elevation in Europe. Glob. Ecol. Biogeogr. 26, 690–699 (2017).
Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (2004).
Weigelt, P. & Kreft, H. Quantifying island isolation—insights from global patterns of insular plant species richness. Ecography 36, 417–429 (2013).
Koske, R., Gemma, J. & Flynn, T. Mycorrhizae in Hawaiian angiosperms: a survey with implications for the origin of the native flora.Am. J. Bot. 79, 853–862 (1992).
Vellinga, E. C., Wolfe, B. E. & Pringle, A. Global patterns of ectomycorrhizal introductions. New Phytol. 181, 960–973 (2009).
Cornelissen, J., Aerts, R., Cerabolini, B., Werger, M. & Van Der Heijden, M. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 129, 611–619 (2001).
Powell, J. R., Riley, R. C. & Cornwell, W. Relationships between mycorrhizal type and leaf flammability in the Australian flora. Pedobiologia 65, 43–49 (2017).
Van Kleunen, M. et al. Global exchange and accumulation of non-native plants. Nature 525, 100–103 (2015).
Weigelt, P., König, C. & Kreft, H. GIFT- A Global Inventory of Floras and Traits; https://doi.org/10.1101/535005 (2019).
Van Kleunen, M. et al. Global exchange and accumulation of non-native plants.Nature 525, 100–103 (2015).
Byng, J. W. et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 181, 1–20 (2016).
Gerdemann, J. Vesicular-arbuscular mycorrhiza and plant growth. Annu. Rev. Phytopathol. 6, 397–418 (1968).
Karger, D. N. et al. Climatologies at high resolution for the Earth’s land surface areas. Sci. Data 4, 170122 (2017).
Danielson, J. J. & Gesch, D. B. Global Multi-resolution Terrain Elevation Data 2010 (GMTED2010) Report 2331-1258 (US Geological Survey, 2011).
Socioeconomic Data and Applications Center (SEDAC).Gridded Population of the World, (GPW), v3; https://doi.org/10.7927/H4639MPP (Center for International Earth Science Information Network, 2015).
Tuanmu, M. N. & Jetz, W. A global 1‐km consensus land‐cover product for biodiversity and ecosystem modelling. Glob. Ecol. Biogeogr. 23, 1031–1045 (2014).
Kueffer, C. et al. A global comparison of plant invasions on oceanic islands. Perspect. Plant Ecol. Evol. Syst. 12, 145–161 (2010).
Triantis, K. A., Economo, E. P., Guilhaumon, F. & Ricklefs, R. E. Diversity regulation at macro‐scales: species richness on oceanic archipelagos. Glob. Ecol. Biogeogr. 24, 594–605 (2015).
Crase, B., Liedloff, A. C. & Wintle, B. A. A new method for dealing with residual spatial autocorrelation in species distribution models. Ecography 35, 879–888 (2012).
Bivand, R. & Piras, G. Comparing Implementations of Estimation Methods for Spatial Econometrics (American Statistical Association, Alexandria, VA, USA, 2015).
R Core Team. R: A language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria. 2016).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Acknowledgements
We acknowledge W. Jetz, D. Sax, A. Mehring and M. Heard for introducing us to collaborators, and S. Queenborough for statistical support at the start of the project. We also acknowledge support from the US NSF (DEB-1556664 to J.D.B., P.S. and C.S.D.), National Geographical Society (WW-036ER-17 to C.S.D.), German DFG (MvK: project 264740629; MW project FZT 118), Czech Centre of Excellence Plant Diversity Analysis and Synthesis Centre (14–36079G to J.P. and P.P.), Czech Academy of Sciences (RVO 67985939 to J.P. and P.P.) and Austrian Science Foundation (I2086B16 to F.E.).
Author information
Authors and Affiliations
Contributions
C.S.D. and J.D.B. designed the study. C.S.D. and J.D.B. led the statistical analysis and writing, with continued contributions from P.W. and H.K. C.S.D. collected all of the mycorrhizal data. All other authors collected the remaining plant and environmental data and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 3, Supplementary Figures 1–7 and Supplementary Note 1
Supplementary Table 2
Plant family with assigned corresponding AMF status and reference(s) cited. This table specifies consensus proportions of known plants within each family that are either mycorrhizal, non-mycorrhizal or ambiguous (AMNM). This is done first for each reference separately and then as an average (used in this study) across available references for each mycorrhizal status.
Supplementary Table 4
Summary of proportions of mycorrhizal and non-mycorrhizal species per region for both native and naturalized plant species. Proportions of mycorrhizal and non-mycorrhizal species within native and naturalized species per region along with the latitude and longitude of the regions’ mass centroids before subsetting or exclusion of zero count data.
Rights and permissions
About this article
Cite this article
Delavaux, C.S., Weigelt, P., Dawson, W. et al. Mycorrhizal fungi influence global plant biogeography. Nat Ecol Evol 3, 424–429 (2019). https://doi.org/10.1038/s41559-019-0823-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-019-0823-4
This article is cited by
-
Mutualisms weaken the latitudinal diversity gradient among oceanic islands
Nature (2024)
-
Invasive palms and WWII damaged an island paradise. Could fungi help to restore it?
Nature (2023)
-
Species diversity of arbuscular mycorrhizal but not ectomycorrhizal plants decreases with habitat loss due to environmental filtering
Plant and Soil (2023)
-
Mycorrhizal feedbacks influence global forest structure and diversity
Communications Biology (2023)
-
Nitrogen-fixing symbiotic bacteria act as a global filter for plant establishment on islands
Communications Biology (2022)