Abstract
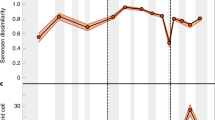
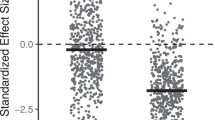
A long-running debate over the affinities of the Neoproterozoic ‘Ediacara biota’ has led to contrasting interpretations of Ediacaran ecosystem complexity. A ‘simple’ model assumes that most, if not all, Ediacaran organisms shared similar basic ecologies. A contrasting ‘complex’ model suggests that the Ediacara biota more likely represent organisms from a variety of different positions on the eukaryotic tree and thus occupied a wide range of different ecologies. We perform a quantitative test of Ediacaran ecosystem complexity using rank abundance distributions (RADs). We show that the Ediacara biota formed complex-type communities throughout much of their stratigraphic range and thus likely comprised species that competed for different resources and/or created niche for others (‘ecosystem engineers’). One possible explanation for this pattern rests in the recent inference of multiple metazoan-style feeding modes among the Ediacara biota; in this scenario, different Ediacaran groups/clades were engaged in different methods of nutrient collection and thus competed for different resources. This result illustrates that the Ediacara biota may not have been as bizarre as it is sometimes suggested, and provides an ecological link with the animal-dominated benthic ecosystems of the Palaeozoic era.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All R code and data are provided in the online supplementary materials.
References
McMenamin, M. A. S. The Garden of Ediacara. PALAIOS 1, 178–182 (1986).
Bush, A. M., Bambach, R. K. & Erwin, D. H. in Quantifying the Evolution of Early Life Topics in Geobiology 1st edn (eds Laflamme, M. et al.) 111–133 (Springer, Berlin, 2011).
Laflamme, M., Darroch, S. A. F., Tweedt, S., Peterson, K. J. & Erwin, D. H. The end of the Ediacara biota: extinction, biotic replacement, or Cheshire Cat?. Gondwana Res. 23, 558–573 (2013).
Schiffbauer, J. D. et al. The latest Ediacaran wormworld fauna: setting the ecological stage for the Cambrian explosion. GSA Today 26, 4–11 (2016).
Erwin, D. H. et al. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097 (2011).
Erwin, D. H. & Valentine, J. W. The Cambrian Explosion: The Construction of Animal Biodiversity. (Roberts & Company: Greenwood Village, 2013).
Rahman, I. A., Darroch, S. A. F., Racicot, R. A. & Laflamme, M. Suspension feeding in the enigmatic Ediacaran organism Tribrachidium demonstrates complexity of Neoproterozoic ecosystems. Sci. Adv. 1, e1500800 (2015).
Darroch, S. A. F., Rahman, I. A., Gibson, B., Racicot, R. A. & Laflamme, M. Inference of facultative mobility in the enigmatic Ediacaran organism Parvancorina. Biol. Lett. 13, 20170033 (2017).
Paterson, J. R., Gehling, J. G., Droser, M. L. & Bicknell, R. D. Rheotaxis in the Ediacaran epibenthic organism Parvancorina from South Australia. Sci. Rep. 7, 45539 (2017).
Wagner, P. J., Kosnik, M. A. & Lidgard, S. Abundance distributions imply elevated complexity of post-Paleozoic marine ecosystems. Science 314, 1289–1292 (2006).
Laflamme, M., Gehling, J. G. & Droser, M. L. Deconstructing an Ediacaran frond: three-dimensional preservation of Arborea from Ediacara, South Australia. J. Paleontol. 92, 323–335 (2018).
Mitchell, E. G. & Butterfield, N. J. Spatial analyses of Ediacaran communities at Mistaken Point. Paleobiology 44, 40–57 (2018).
Gray, J. S. in Organization of Communities Past and Present (eds Gee, J. H. R. & Gillier, P. S.) 53–67 (Blackwell, Oxford, 1987).
Root, R. B. The niche exploitation pattern of the blue-gray gnatcatcher. Ecol. Monogr. 37, 317–350 (1967).
Fisher, R. A., Corbet, A. S. & Williams, C. B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 12, 42–58 (1943).
Hubbell, S. P. A unified theory of biogeography and relative species abundance and its application to tropical rain forests and coral reefs. Coral Reefs 16(Suppl 1), S9–S21 (1997).
Frontier, S. in Oceanography and Marine Biology: An Annual Review (ed. Barnes, M.) 253–312 (Aberdeen Univ. Press, Aberdeen,1985).
Laland, K. N., Odling-Smee, F. J. & Feldman, M. W. Evolutionary consequences of niche construction and their implications for ecology. Proc. Natl Acad. Sci. USA 96, 10242–10247 (1999).
Kosnik, M. A. & Wagner, P. J. Effects of taxon abundance distributions on expected numbers of sampled taxa. Evol. Ecol. Res. 8, 195–211 (2006).
Xiao, S. & Laflamme, M. On the eve of animal radiation: phylogeny, ecology and evolution of the Ediacara biota. Trends Ecol. Evol. 24, 31–40 (2009).
Tucker, C. M., Davies, T. J., Cadotte, M. W. & Pearse, W. D. On the relationship between phylogenetic diversity and trait diversity. Ecology 99, 1473–1479 (2018).
Laflamme, M., Xiao, S. & Kowalewski, M. From the cover: osmotrophy in modular Ediacara organisms. Proc. Natl Acad. Sci. USA 106, 14438–14443 (2009).
Sperling, E. A., Peterson, K. J. & Laflamme, M. Rangeomorphs, Thectardis (Porifera?) and dissolved organic carbon in the Ediacaran oceans. Geobiology 9, 24–33 (2011).
Glaessner, M. F. in Treatise on Invertebrate Paleontology. Part A (eds Robison, R. A. & Teichert, C.) A79–A118 (Univ. Kansas Press, Boulder, 1979).
Jenkins, R. J. F. The enigmatic Ediacaran (Late Precambrian) genus Rangea and related forms. Paleobiology 11, 336–355 (1985).
Gehling, J. G. The Case for Ediacaran Fossil Roots to the Metazoan Tree Vol. 20, 181–224 (Geological Society of India, Bangalore, 1991).
Antcliffe, J. B. & Brasier, M. D. Charnia at 50: developmental models for Ediacaran fronds. Palaeontology 51, 11–26 (2008).
Budd, G. E. & Jensen, S. The origin of the animals and a ‘Savannah’ hypothesis for early bilaterian evolution. Biol. Rev. Camb. Philos. Soc. 92, 446–473 (2017).
Dececchi, T. A., Narbonne, G. M., Greentree, C. & Laflamme, M. Relating Ediacaran fronds. Paleobiology 43, 171–180 (2017).
Sperling, E. A. & Vinther, J. A placozoan affinity for Dickinsonia and the evolution of late Proterozoic metazoan feeding modes. Evol. Dev. 12, 201–209 (2010).
Droser, M. L., Tarhan, L. G. & Gehling, J. G. The rise of animals in a changing environment: global ecological innovation in the Late Ediacaran. Annu. Rev. Earth. Planet. Sci. 45, 593–617 (2017).
Droser, M. L. & Gehling, J. G. The advent of animals: the view from the Ediacaran. Proc. Natl Acad. Sci. USA 112, 4865–4870 (2015).
Darroch, A. F., Laflamme, M. & Clapham, M. E. Population structure of the oldest known macroscopic communities from Mistaken Point, Newfoundland. Paleobiology 39, 591–608 (2013).
Clapham, M. E., Narbonne, G. M. & Gehling, J. G. Paleoecology of the oldest known animal communities: Ediacaran assemblages at Mistaken Point, Newfoundland. Paleobiology 29, 527–544 (2003).
Gehling, J. G. & Droser, M. L. How well do fossil assemblages of the Ediacara biota tell time?. Geology 41, 447–450 (2013).
Zakrevskaya, M. Paleoecological reconstruction of the Ediacaran benthic macroscopic communities of the White Sea (Russia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 410, 27–38 (2014).
Coutts, F. J., Gehling, J. G. & García-Bellido, D. C. How diverse were early animal communities? An example from Ediacara Conservation Park, Flinders Ranges, South Australia. Alcheringa 40, 407–421 (2016).
Droser, M. L., Gehling, J. G. & Jensen, S. R. Assemblage palaeoecology of the Ediacara biota: the unabridged edition? Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 131–1457 (2006).
Darroch, S. A. F. et al. Biotic replacement and mass extinction of the Ediacara biota. Proc. Biol. Sci. 282, 20151003 (2015).
Boag, T. H., Darroch, S. A. F. & Laflamme, M. Ediacaran distributions in space and time: testing assemblage concepts of earliest macroscopic body fossils. Paleobiology 42, 574–594 (2016).
Fedonkin, M. A. & Waggoner, B. M. The Late Precambrian fossil Kimberella is a mollusc-like bilaterian organism. Nature 388, 868–871 (1997).
Gehling, J. G., Runnegar, B. N. & Droser, M. L. Scratch traces of large Ediacara bilaterian animals. J. Paleontol. 88, 284–298 (2014).
Ivantsov, A. Y. New reconstruction of Kimberella, problematic Vendian metazoan. Paleontol. J. 43, 601–611 (2009).
Ivantsov, A. Y. & Malakhovskaya, Y. E. Giant traces of Vendian animals. Doklady Earth Sci. 385A, 618–622 (2002).
Liu, A. G., Kenchington, C. G. & Mitchell, E. G. Remarkable insights into the paleoecology of the Avalonian Ediacaran macrobiota. Gondwana Res. 27, 1355–1380 (2015).
Jensen, S. & Runnegar, B. N. A complex trace fossil from the Spitskop Member (terminal Ediacaran–? Lower Cambrian) of southern Namibia. Geol. Mag. 142, 561–569 (2005).
Mángano, M. G. & Buatois, L. A. Decoupling of body-plan diversification and ecological structuring during the Ediacaran–Cambrian transition: evolutionary and geobiological feedbacks. Proc. Biol. Sci. 281, 20140038 (2014).
Buatois, L. A., Wisshak, M., Wilson, M. A. & Mángano, M. G. Categories of architectural designs in trace fossils: a measure of ichnodisparity. Earth Sci. Rev. 164, 102–181 (2017).
Smith, E. F. et al. The end of the Ediacaran: two new exceptionally preserved body fossil assemblages from Mount Dunfee, Nevada, USA. Geology 44, 911–914 (2016).
Darroch, S. A. F., Smith, E. F., Laflamme, M. & Erwin, D. H. Ediacaran extinction and Cambrian explosion. Trends Ecol. Evol. 33, 653–663 (2018).
Gray, J. S. Pollution-induced changes in populations. Phil. Trans. R. Soc. Lond. B 286, 545–561 (1979).
Hamer, K. C., Hill, J. K., Lace, L. A. & Langan, A. M. Ecological and biogeographical effects of forest disturbance on tropical butterflies of Sumba, Indonesia. J. Biogeogr. 24, 67–75 (1997).
Hill, J. K., Hamer, K. C., Lace, L. A. & Banham, W. M. T. Effects of selective logging on tropical forest butterflies on Buru, Indonesia. J. Appl. Ecol. 32, 754–760 (1995).
Mouillot, D. & Lepretre, A. Introduction of relative abundance distribution (RAD) indices, estimated from the rank-frequency diagrams (RFD), to assess changes in community diversity. Environ. Monit. Assess. 63, 279–295 (2000).
McElwain, J. C., Wagner, P. J. & Hesselbo, S. P. Fossil plant relative abundances indicate sudden loss of Late Triassic biodiversity in Greenland. Science 324, 1554–1556 (2009).
Liu, A. G., Mcllroy, D. & Brasier, M. D. First evidence for locomotion in the Ediacara biota from the 565 Ma Mistaken Point Formation, Newfoundland. Geology 38, 123–126 (2010).
Buatois, L. A. & Mángano, M. G. Ichnology: Organism–Substrate Interactions in Space and Time (Cambridge Univ. Press, Cambridge, 2011).
Bottjer, D. J. & Jablonski, D. Paleoenvironmental patterns in the evolution of post-Paleozoic benthic marine invertebrates. PALAIOS 3, 540–560 (1988).
Brocks, J. J. et al. The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548, 578–581 (2017).
Butterfield, N. J. Early evolution of the Eukaryota. Palaeontology 58, 5–17 (2015).
Mitchell, E. G., Kenchington, C. G., Liu, A. G., Matthews, J. J. & Butterfield, N. J. Reconstructing the reproductive mode of an Ediacaran macro-organism. Nature 524, 343–346 (2015).
Gehling, J. G. Microbial mats in terminal Proterozoic siliciclastics: Ediacaran death masks. PALAIOS 14, 40–57 (1999).
Budd, G. E. & Jensen, S. A critical reappraisal of the fossil record of the bilaterian phyla. Biol. Rev. Camb. Philos. Soc. 75, 253–295 (2000).
Gibson, B. M., Schiffbauer, J. D. & Darroch, S. A. F. Ediacaran-style decay experiments using mollusks and sea anemones. PALAIOS 33, 185–203 (2018).
Gehling, J. G. Sequence stratigraphic context of the Ediacara Member, Rawnsley Quartzite, South Australia: a taphonomic window into the Neoproterozoic biosphere. Precambrian Res. 100, 65–95 (2000).
Tarhan, L. G., Droser, M. L. & Gehling, J. G. Taphonomic controls on Ediacaran diversity: uncovering the holdfast origin of morphologically variable enigmatic structures. PALAIOS 25, 823–830 (2010).
Burzynski, G. & Narbonne, G. M. The discs of Avalon: relating discoid fossils to frondose organisms in the Ediacaran of Newfoundland, Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 434, 34–45 (2015).
Sugiura, N. Further analysis of the data by Akaike’s information criterion and the finite corrections. Commun. Stat. Theory Methods A7, 13–26 (1978).
Liu, A. G., McIlroy, D., Antcliffe, J. B. & Brasier, M. D. Effaced preservation in the Ediacara biota and its implications for the early macrofossil record. Palaeontology 54, 607–630 (2011).
Plotnick, R. E. & Sepkoski, J. Jr. A multiplicative multifractal model for originations and extinctions. Paleobiology 27, 126–139 (2001)..
Acknowledgements
S.A.F.D. was funded by a Smithsonian Institution Peter Buck Postdoctoral Fellowship. M.L. and S.A.F.D. also acknowledge generous funding from a National Geographic Research and Exploration Grant (9241-13), which allowed collection of Ediacaran population data from Namibia. This is Paleobiology Database Publication 318.
Author information
Authors and Affiliations
Contributions
S.A.F.D. and M.L. collected and compiled the data. P.J.W. and S.A.F.D. performed the analyses. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary tables, figures and references
Supplementary Dataset 1
Excel file detailing the taxonomic composition (and assignments) for all assemblages used in analyses
Supplementary Dataset 2
R code written and used by the authors to calculate RAD fits for Ediacaran datasets
Supplementary dataset 3
txt file for use with R code, providing relative abundances of Ediacaran taxa within studied assemblages
Supplementary dataset 4
txt file for use with R code, providing the names of assemblages used in analyses
Rights and permissions
About this article
Cite this article
Darroch, S.A.F., Laflamme, M. & Wagner, P.J. High ecological complexity in benthic Ediacaran communities. Nat Ecol Evol 2, 1541–1547 (2018). https://doi.org/10.1038/s41559-018-0663-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0663-7
This article is cited by
-
Pentaradial eukaryote suggests expansion of suspension feeding in White Sea-aged Ediacaran communities
Scientific Reports (2021)
-
Viewing the Ediacaran biota as a failed experiment is unhelpful
Nature Ecology & Evolution (2019)