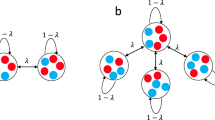
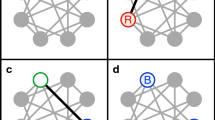
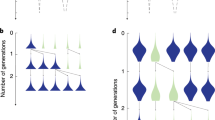
Abstract
Ecological and evolutionary dynamics of communities are inexorably intertwined. The ecological state determines the fate of newly arising mutants, and mutations that increase in frequency can reshape the ecological dynamics. Evolutionary game theory and its extensions within adaptive dynamics have been the mathematical frameworks for understanding this interplay, leading to notions such as evolutionarily stable states (ESS) in which no mutations are favoured, and evolutionary branching points near which the population diversifies. A central assumption behind these theoretical treatments has been that mutations are rare so that the ecological dynamics has time to equilibrate after every mutation. A fundamental question is whether qualitatively new phenomena can arise when mutations are frequent. Here, we describe an adaptive diversification process that robustly leads to complex ESS, despite the fact that such communities are unreachable through a step-by-step evolutionary process. Rather, the system as a whole tunnels between collective states over a short timescale. The tunnelling rate is a sharply increasing function of the rate at which mutations arise in the population. This makes the emergence of ESS communities virtually impossible in small populations, but generic in large ones. Moreover, communities emerging through this process can spatially spread as single replication units that outcompete other communities. Overall, this work provides a qualitatively new mechanism for adaptive diversification and shows that complex structures can generically evolve even when no step-by-step evolutionary path exists.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schoener, T. W. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429 (2011).
Pelletier, F., Garant, D. & Hendry, A. P. Eco-evolutionary dynamics. Phil. Trans. R. Soc. Lond. B 364, 1483–1489 (2009).
Haloin, J. R. & Strauss, S. Y. Interplay between ecological communities and evolution. Ann. NY Acad. Sci. 1133, 87–125 (2008).
Weber, M. G., Wagner, C. E., Best, R. J., Harmon, L. J. & Matthews, B. Evolution in a community context: on integrating ecological interactions and macroevolution. Trends Ecol. Evol. 32, 291–304 (2017).
Post, D. M. & Palkovacs, E. P. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640 (2009).
Sanchez, A. & Gore, J. Feedback between population and evolutionary dynamics determines the fate of social microbial populations. PLoS Biol. 11, e1001547 (2013).
Rainey, P. B. & Travisano, M. Adaptive radiation in a heterogeneous environment. Nature 394, 69–72 (1998).
Gómez, P. & Buckling, A. Real-time microbial adaptive diversification in soil. Ecol. Lett. 16, 650–655 (2013).
Callahan, B. J., Fukami, T. & Fisher, D. S. Rapid evolution of adaptive niche construction in experimental microbial populations. Evolution 68, 3307–3316 (2014).
Koeppel, A. F. et al. Speedy speciation in a bacterial microcosm: new species can arise as frequently as adaptations within a species. ISME J. 7, 1080–1091 (2013).
Hiltunen, T., Virta, M. & Laine, A.-L. Antibiotic resistance in the wild: an eco-evolutionary perspective. Phil. Trans. R. Soc. Lond. B 372, 20160039 (2017).
Fischbach, M. A., Walsh, C. T. & Clardy, J. The evolution of gene collectives: how natural selection drives chemical innovation. Proc. Natl Acad. Sci. USA 105, 4601–4608 (2008).
Celiker, H. & Gore, J. Clustering in community structure across replicate ecosystems following a long-term bacterial evolution experiment. Nat. Commun. 5, 4643 (2014).
Cordero, O. X. & Polz, M. F. Explaining microbial genomic diversity in light of evolutionary ecology. Nat. Rev. Microbiol. 12, 263–273 (2014).
Stein, R. R. et al. Ecological modeling from time-series inference: insight into dynamics and stability of intestinal microbiota. PLoS Comput. Biol. 9, e1003388 (2013).
Coyte, K. Z., Schluter, J. & Foster, K. R. The ecology of the microbiome: networks, competition, and stability. Science 350, 663–666 (2015).
Friedman, J., Higgins, L. M. & Gore, J. Community structure follows simple assembly rules in microbial microcosms. Nat. Ecol. Evol. 1, 0109 (2017).
Yoshida, T., Jones, L. E., Ellner, S. P., Fussmann, G. F. & Hairston, N. G. Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424, 303–306 (2003).
Lawrence, D. et al. Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 10, e1001330 (2012).
Herron, M. D. & Doebeli, M. Parallel evolutionary dynamics of adaptive diversification in Escherichia coli. PLoS Biol. 11, e1001490 (2013).
Harrington, K. I. & Sanchez, A. Eco-evolutionary dynamics of complex social strategies in microbial communities. Commun. Integr. Biol. 7, e28230 (2014).
Good, B. H., McDonald, M. J., Barrick, J. E., Lenski, R. E. & Desai, M. M. The dynamics of molecular evolution over 60,000 generations. Nature 551, 45–50 (2017).
Maynard Smith, J. & Price, G. R. The logic of animal conflict. Nature 246, 15–18 (1973).
Maynard Smith, J. Evolution and the Theory of Games (Cambridge Univ. Press, Cambridge, 1982).
Levin, S. A. & Muller-Landau, H. C. The emergence of diversity in plant communities. C. R. Acad. Sci. III 323, 129–139 (2000).
Conlin, P. L., Chandler, J. R. & Kerr, B. Games of life and death: antibiotic resistance and production through the lens of evolutionary game theory. Curr. Opin. Microbiol. 21, 35–44 (2014).
Boyd, R. & Lorberbaum, J. P. No pure strategy is evolutionarily stable in the repeated prisoner’s dilemma game. Nature 327, 58–59 (1987).
Nowak, M. A. & Sigmund, K. Evolutionary dynamics of biological games. Science 303, 793–799 (2004).
Geritz, S. A. H., Kisdi, E., Meszena, G. & Metz, J. A. J. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol. Ecol. 12, 35–57 (1998).
Diekmann O. A Beginner’s Guide to Adaptive Dynamics Mathematical Modelling of Population Dynamics Vol. 63 (ed. Rudnicki, R.) 47–86 (Banach Center Publications, Warsaw, 2004).
Brännström, Johansson, J. & von Festenberg, N. The hitchhikeras guide to adaptive dynamics. Games 4, 304–328 (2013).
Nowak, M. An evolutionarily stable strategy may be inaccessible. J. Theor. Biol. 142, 237–241 (1990).
Boldin, B. & Diekmann, O. An extension of the classification of evolutionarily singular strategies in adaptive dynamics. J. Math. Biol. 69, 905–940 (2013).
Doebeli, M. Adaptive Diversification (MPB-48) (Princeton Univ. Press, Princeton, NJ, 2011).
Gerrish, P. J. & Lenski, R. E. The fate of competing beneficial mutations in an asexual population. Genetica 102/103, 127–144 (1998).
Hegreness, M., Shoresh, N., Hartl, D. & Kishony, R. An equivalence principle for the incorporation of favorable mutations in asexual populations. Science 311, 1615–1617 (2006).
Iwasa, Y., Michor, F. & Nowak, M. A. Stochastic tunnels in evolutionary dynamics. Genetics 166, 1571–1579 (2004).
Desai, M. M. & Fisher, D. S. Beneficial mutation selection balance and the effect of linkage on positive selection. Genetics 176, 1759–1798 (2007).
Weissman, D. B., Desai, M. M., Fisher, D. S. & Feldman, M. W. The rate at which asexual populations cross fitness valleys. Theor. Popul. Biol. 75, 286–300 (2009).
Weissman, D. B., Feldman, M. W. & Fisher, D. S. The rate of fitness-valley crossing in sexual populations. Genetics 186, 1389–1410 (2010).
Martens, E. A Kostadinov, R, Maley, C. C & Hallatschek, O. Spatial structure increases the waiting time for cancer. New J. Phys. 13, 115014 (2011).
Vetsigian, K. Diverse modes of eco-evolutionary dynamics in communities of antibiotic-producing microorganisms. Nat. Ecol. Evol. 1, 0189 (2017).
Weinreich, D. M. & Chao, L. Rapid evolutionary escape by large populations from local fitness peaks is likely in nature. Evolution 59, 1175–1182 (2005).
Kerr, B., Riley, M. A., Feldman, M. W. & Bohannan, B. J. M. Local dispersal promotes biodiversity in a real-life game of rock–paper–scissors. Nature 418, 171–174 (2002).
Reichenbach, T., Mobilia, M. & Frey, E. Mobility promotes and jeopardizes biodiversity in rock–paper–scissors games. Nature 448, 1046–1049 (2007).
Karthika, S., Radhakrishnan, T. K. & Kalaichelvi, P. A review of classical and nonclassical nucleation theories. Cryst. Growth Des. 16, 6663–6681 (2016).
Babichenko, Y. & Rubinstein, A. Communication complexity of approximate Nash equilibria. In Proc. 49th Annual ACM SIGACT Symposium on Theory of Computing (STOC 2017) 878–889 (ACM Press, 2017).
Kimura, M. & Ohta, T. The average number of generations until fixation of a mutant gene in a finite population. Genetics 61, 763–771 (1969).
Acknowledgements
This work was supported by the Simons Foundation, Targeted Grant in the Mathematical Modeling of Living Systems Award 342039 and National Science Foundation Grant DEB 1457518. This research was performed using the compute resources and assistance of the University of Wisconsin-Madison Center for High Throughput Computing in the Department of Computer Sciences. We thank S. A. Levin and D. Weissman for insightful discussions.
Author information
Authors and Affiliations
Contributions
S.E.K. and K.V. designed the study, analysed the data, performed the simulations and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures 1–10
Rights and permissions
About this article
Cite this article
Kotil, S.E., Vetsigian, K. Emergence of evolutionarily stable communities through eco-evolutionary tunnelling. Nat Ecol Evol 2, 1644–1653 (2018). https://doi.org/10.1038/s41559-018-0655-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0655-7
This article is cited by
-
Eco-evolutionary feedback as a driver of periodic state shifts in tri-trophic food chains
Evolutionary Ecology (2023)
-
Contingent evolution of alternative metabolic network topologies determines whether cross-feeding evolves
Communications Biology (2020)
-
Seasonal payoff variations and the evolution of cooperation in social dilemmas
Scientific Reports (2019)
-
Spatial eco-evolutionary feedbacks mediate coexistence in prey-predator systems
Scientific Reports (2019)
-
Fast evolution unlocks forbidden communities
Nature Ecology & Evolution (2018)