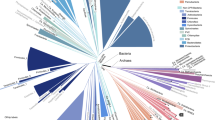
Abstract
Numerous studies have estimated plant and animal diversification dynamics; however, no comparable rigorous estimates exist for bacteria—the most ancient and widespread form of life on Earth. Here, we analyse phylogenies comprising up to 448,112 bacterial lineages to reconstruct global bacterial diversification dynamics. To handle such large phylogenies, we developed methods based on the statistical properties of infinitely large trees. We further analysed sequencing data from 60 environmental studies to determine the fraction of extant bacterial diversity missing from the phylogenies—a crucial parameter for estimating speciation and extinction rates. We estimate that there are about 1.4–1.9 million extant bacterial lineages when lineages are defined by 99% similarity in the 16S ribosomal RNA gene, and that bacterial diversity has been continuously increasing over the past 1 billion years (Gyr). Recent bacterial extinction rates are estimated at 0.03–0.05 per lineage per million years (lineage–1 Myr–1), and are only slightly below estimated recent bacterial speciation rates. Most bacterial lineages ever to have inhabited this planet are estimated to be extinct. Our findings disprove the notion that bacteria are unlikely to go extinct, and provide a valuable perspective on the evolutionary history of a domain of life with a sparse and cryptic fossil record.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Falkowski, P. G., Fenchel, T. & Delong, E. F. The microbial engines that drive Earth’s biogeochemical cycles. Science 320, 1034–1039 (2008).
Fischer, W. W., Hemp, J. & Johnson, J. E. Evolution of oxygenic photosynthesis. Annu. Rev. Earth Planet. Sci. 44, 647–683 (2016).
Raup, D. M. & Sepkoski, J. J. Mass extinctions in the marine fossil record. Science 215, 1501–1503 (1982).
Signor, P. W. Biodiversity in geological time. Am. Zool. 34, 23–32 (1994).
McElwain, J. C. & Punyasena, S. W. Mass extinction events and the plant fossil record. Trends Ecol. Evol. 22, 548–557 (2007).
Nee, S., May, R. M. & Harvey, P. H. The reconstructed evolutionary process. Phil. Trans. R. Soc. Lond. B 344, 305–311 (1994).
Nee, S., Holmes, E. C., May, R. M. & Harvey, P. H. Extinction rates can be estimated from molecular phylogenies. Phil. Trans. R. Soc. Lond. B 344, 77–82 (1994).
Sanderson, M. J. & Donoghue, M. J. Reconstructing shifts in diversification rates on phylogenetic trees. Trends Ecol. Evol. 11, 15–20 (1996).
Morlon, H. Phylogenetic approaches for studying diversification. Ecol. Lett. 17, 508–525 (2014).
Morlon, H., Kemps, B. D., Plotkin, J. B. & Brisson, D. Explosive radiation of a bacterial species group. Evolution 66, 2577–2586 (2012).
Lorén, J. G., Farfán, M. & Fusté, M. C. Molecular phylogenetics and temporal diversification in the genus Aeromonas based on the sequences of five housekeeping genes. PLoS. ONE 9, 1–15 (2014).
Lebreton, F. et al. Tracing the Enterococci from paleozoic origins to the hospital. Cell 169, 849–861 (2017).
Gubry-Rangin, C. et al. Coupling of diversification and pH adaptation during the evolution of terrestrial Thaumarchaeota. Proc. Natl Acad. Sci. USA 112, 9370–9375 (2015).
Marin, J., Battistuzzi, F. U., Brown, A. C. & Hedges, S. B. The timetree of prokaryotes: new insights into their evolution and speciation. Mol. Biol. Evol. 34, 437–446 (2017).
Stadler, T. On incomplete sampling under birth–death models and connections to the sampling-based coalescent. J. Theor. Biol. 261, 58–66 (2009).
Hug, L. A. et al. A new view of the tree of life. Nat. Microbiol. 1, 16048 (2016).
Schopf, J. W. Disparate rates, differing fates: tempo and mode of evolution changed from the Precambrian to the Phanerozoic. Proc. Natl Acad. Sci. USA 91, 6735–6742 (1994).
Dykhuizen, D. E. Santa rosalia revisited: why are there so many species of bacteria? Antonie Van Leeuwenhoek 73, 25–33 (1998).
Butterfield, N. Macroevolution and macroecology through deep time. Palaeontology 50, 41–55 (2007).
Schopf, J. W. et al. Sulfur-cycling fossil bacteria from the 1.8-Ga duck creek formation provide promising evidence of evolution’s null hypothesis. Proc. Natl Acad. Sci. USA 112, 2087–2092 (2015).
Weinbauer, M. G. & Rassoulzadegan, F. Extinction of microbes: evidence and potential consequences. Endang. Species Res. 3, 205–215 (2007).
Höhna, S., Stadler, T., Ronquist, F. & Britton, T. Inferring speciation and extinction rates under different sampling schemes. Mol. Biol. Evol. 28, 2577–2589 (2011).
Stackebrandt, E. & Ebers, J. Taxonomic parameters revisited: tarnished gold standards. Microbiol. Today 33, 152–155 (2006).
Edgar, R. C. Updating the 97% identity threshold for 16S ribosomal RNA OTUs. Bioinformatics 34, 2371–2375 (2018).
Sanmartn, I. & Meseguer, A. S. Extinction in phylogenetics and biogeography: from timetrees to patterns of biotic assemblage. Front. Genet. 7, 35 (2016).
Stadler, T. Mammalian phylogeny reveals recent diversification rate shifts. Proc. Natl Acad. Sci. USA 108, 6187–6192 (2011).
Silvestro, D., Schnitzler, J. & Zizka, G. A Bayesian framework to estimate diversification rates and their variation through time and space. BMC Evol. Biol. 11, 311 (2011).
Stadler, T. How can we improve accuracy of macroevolutionary rate estimates? Syst. Biol. 62, 321–329 (2013).
Marshall, C. R. Five palaeobiological laws needed to understand the evolution of the living biota. Nat. Ecol. Evol. 1, 165 (2017).
Schloss, P. D., Girard, R. A., Martin, T., Edwards, J. & Thrash, J. C. Status of the archaeal and bacterial census: an update. mBio 7, e00201-16 (2016).
Locey, K. J. & Lennon, J. T. Scaling laws predict global microbial diversity. Proc. Natl Acad. Sci. USA 113, 5970–5975 (2016).
Thompson, L. R. et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551, 457–463 (2017).
Glöckner, F. O. et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 261, 169–176 (2017).
Krebs, C. J. Ecological Methodology (Benjamin Cummings, San Francisco, CA, 1999).
Zanne, A. E. et al. Three keys to the radiation of angiosperms into freezing environments. Nature 505, 89–99 (2014).
Jetz, W. et al. Global distribution and conservation of evolutionary distinctness in birds. Curr. Biol. 24, 919–930 (2014).
Welch, R. A. et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl Acad. Sci. USA 99, 17020–17024 (2002).
Shapiro, B. J. & Polz, M. F. Ordering microbial diversity into ecologically and genetically cohesive units. Trends Microbiol. 22, 235–247 (2014).
Xie, S., Pancost, R. D., Yin, H., Wang, H. & Evershed, R. P. Two episodes of microbial change coupled with Permo/Triassic faunal mass extinction. Nature 434, 494–497 (2005).
Gibbons, S. M. et al. Evidence for a persistent microbial seed bank throughout the global ocean. Proc. Natl Acad. Sci. USA 110, 4651–4655 (2013).
Pimm, S. L. et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014).
Kim, M., Oh, H.-S., Park, S.-C. & Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 64, 346–351 (2014).
Straub, T. J. & Zhaxybayeva, O. A null model for microbial diversification. Proc. Natl Acad. Sci. USA 114, E5414–E5423 (2017).
Whitman, W. B., Coleman, D. C. & Wiebe, W. J. Prokaryotes: the unseen majority. Proc. Natl Acad. Sci. USA 95, 6578–6583 (1998).
Butterfield, N. J. Proterozoic photosynthesis—a critical review. Palaeontology 58, 953–972 (2015).
Brocks, J. J. & Banfield, J. Unravelling ancient microbial history with community proteogenomics and lipid geochemistry. Nat. Rev. Microbiol. 7, 601–609 (2009).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Parks, D. H. et al. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol. 2, 1533–1542 (2017).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Magoc, T. & Salzberg, S. L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963 (2011).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584 (2016).
Li, W., Fu, L., Niu, B., Wu, S. & Wooley, J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinform. 13, 656–668 (2012).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 26, 1641–1650 (2009).
Rasmussen, B., Fletcher, I. R., Brocks, J. J. & Kilburn, M. R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455, 1101–1104 (2008).
Shih, P. M., Hemp, J., Ward, L. M., Matzke, N. J. & Fischer, W. W. Crown group Oxyphotobacteria postdate the rise of oxygen. Geobiology 15, 19–29 (2017).
Parfrey, L. W., Lahr, D. J. G., Knoll, A. H. & Katz, L. A. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl Acad. Sci. USA 108, 13624–13629 (2011).
Brocks, J. J. et al. Biomarker evidence for green and purple sulphur bacteria in a stratified palaeoproterozoic sea. Nature 437, 866–870 (2005).
Walter, M., Buick, R. & Dunlop, J. Stromatolites 3,400–3,500 Myr old from the North Pole area, Western Australia. Nature 284, 443–445 (1980).
Ryder, G., Koeberl, C. & Mojzsis, S. J. in Origin of the Earth and Moon (eds Canup, R. & Kevin Righter, K.) 475–492 (Univ. Arizona Press, Tucson, AZ, 2000).
Dykhuizen, D. Species numbers in bacteria. Proc. Calif. Acad. Sci. 56, 62–71 (2005).
Fraser, C., Alm, E. J., Polz, M. F., Spratt, B. G. & Hanage, W. P. The bacterial species challenge: making sense of genetic and ecological diversity. Science 323, 741–746 (2009).
Drummond, A. J. & Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (2007).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Britton, T., Anderson, C. L., Jacquet, D., Lundqvist, S. & Bremer, K. Estimating divergence times in large phylogenetic trees. Syst. Biol. 56, 741–752 (2007).
May, R. M. How many species inhabit the earth? Sci. Am. 267, 42–49 (1992).
Mora, C., Tittensor, D. P., Adl, S., Simpson, A. G. & Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 9, e1001127 (2011).
Eastman, J. M., Harmon, L. J., Tank, D. C. & Paradis, E. Congruification: support for time scaling large phylogenetic trees. Methods Ecol. Evol. 4, 688–691 (2013).
Louca, S. & Doebeli, M. Efficient comparative phylogenetics on large trees. Bioinformatics 34, 1053–1055 (2017).
Smith, S. A., Beaulieu, J. M. & Donoghue, M. J. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc. Natl Acad. Sci. USA 107, 5897–5902 (2010).
Kuo, C.-H. & Ochman, H. Inferring clocks when lacking rocks: the variable rates of molecular evolution in bacteria. Biol. Direct 4, 35 (2009).
Day, W. H. E. Optimal algorithms for comparing trees with labeled leaves. J. Classif. 2, 7–28 (1985).
Borg, I., Groenen, P. J. F. & Mair, P. Applied Multidimensional Scaling (Springer, Berlin, 2013).
Ricklefs, R. E. Estimating diversification rates from phylogenetic information. Trends Ecol. Evol. 22, 601–610 (2007).
Ochman, H. & Wilson, A. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J. Mol. Evol. 26, 74–86 (1987).
Moran, N. A., Munson, M. A., Baumann, P. & Ishikawa, H. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc. R. Soc. Lond. B 253, 167–171 (1993).
Scrucca, L. Model-based SIR for dimension reduction. Comput. Stat. Data Anal. 55, 3010–3026 (2011).
Acknowledgements
We thank D. H. Parks for providing the 16S rRNA sequences from MAGs48. S.L. was supported by an NSERC grant and a postdoctoral fellowship from the Biodiversity Research Centre, University of British Columbia. M.W.P., M.D. and L.W.P. were supported by NSERC Discovery Grants. P.M.S. was supported by The Branco Weiss Fellowship – Society in Science. W.W.F. acknowledges support from the Simons Collaboration on the Origins of Life and NASA Exobiology award number NNX16AJ57G.
Author information
Authors and Affiliations
Contributions
S.L., L.W.P. and M.D. conceived the project. S.L. developed the mathematical methods, performed the diversification analyses and wrote the first draft of the manuscript. P.M.S. performed the molecular clock analyses of the BEAST trees, provided the cyanobacterial multigene tree and contributed to the development of the project ideas. All authors helped to interpret the results, advised on methodological improvements and contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures and tables.
Supplementary file 1
Accession numbers and summaries of amplicon sequencing data used to recover de novo OTUs.
Supplementary file 2
Accession numbers and summaries of sequencing data used from the Earth Microbiome Project.
Supplementary file 3
R code used for analysing diversification dynamics, including required input files.
Supplementary file 4
Time trees and undated phylogenetic trees constructed in this study.
Supplementary file 5
Taxonomic classifications of de novo OTUs.
Rights and permissions
About this article
Cite this article
Louca, S., Shih, P.M., Pennell, M.W. et al. Bacterial diversification through geological time. Nat Ecol Evol 2, 1458–1467 (2018). https://doi.org/10.1038/s41559-018-0625-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0625-0
This article is cited by
-
The rates of global bacterial and archaeal dispersal
The ISME Journal (2022)
-
An Ethical Overview of the CRISPR-Based Elimination of Anopheles gambiae to Combat Malaria
Journal of Bioethical Inquiry (2022)
-
Cage and maternal effects on the bacterial communities of the murine gut
Scientific Reports (2021)
-
Timing the evolution of antioxidant enzymes in cyanobacteria
Nature Communications (2021)
-
Microbial mats in the Turks and Caicos Islands reveal diversity and evolution of phototrophy in the Chloroflexota order Aggregatilineales
Environmental Microbiome (2020)