Abstract
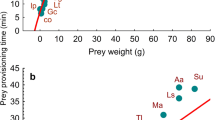
The proportion of time an animal spends actively foraging in a day determines its long-term fitness. Here, we derive a general mathematical model for the scaling of this activity time with body size in consumers. We show that this scaling can change from positive (increasing with size) to negative (decreasing with size) if the detectability and availability of preferred prey sizes is a limiting factor. These predictions are supported by a global dataset on 73 terrestrial carnivore species from 8 families spanning >3 orders of magnitude in size. Carnivores weighing ∼5 kg experience high foraging costs because their diets include significant proportions of relatively small (invertebrate) prey. As a result, they show an increase in activity time with size. This shifts to a negative scaling in larger carnivores as they shift to foraging on less costly vertebrate prey. Our model can be generalized to other classes of terrestrial and aquatic consumers and offers a general framework for mechanistically linking body size to population fitness and vulnerability in consumers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Woodroffe, R. & Ginsberg, J. Edge effects and the extinction of populations inside protected areas. Science 280, 2126–2128 (1998).
Jetz, W., Carbone, C., Fulford, J. & Brown, J. H. The scaling of animal space use. Science 306, 266–268 (2004).
McCain, C. M. & King, S. R. B. Body size and activity times mediate mammalian responses to climate change. Glob. Change Biol. 20, 1760–1769 (2014).
Miller, C. et al. Amur tiger (Panthera tigris altaica) energetic requirements: implications for conserving wild tigers. Biol. Conserv. 170, 120–129 (2014).
Peters, R. The Ecological Implications of Body Size 1st edn (Cambridge Univ. Press, Cambridge, 1983).
Schmidt-Nielsen, K. Scaling: Why is Animal Size so Important? (Cambridge Univ. Press, Cambridge, 1984).
Gorman, M., Mills, M., Raath, J. & Speakman, J. High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 852, 1992–1994 (1998).
Kolokotrones, T., Savage, V., Deeds, E. J. & Fontana, W. Curvature in metabolic scaling. Nature 464, 753–756 (2010).
Speakman, J. R. & Król, E. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746 (2010).
Hudson, L. N., Isaac, N. J. B. & Reuman, D. C. The relationship between body mass and field metabolic rate among individual birds and mammals. J. Anim. Ecol. 82, 1009–1020 (2013).
Weibel, E. R., Bacigalupe, L. D., Schmitt, B. & Hoppeler, H. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respir. Physiol. Neurobiol. 140, 115–132 (2004).
McGill, B. J. & Mittelbach, G. G. An allometric vision and motion model to predict prey encounter rates. Evol. Ecol. Res. 8, 691–701 (2006).
Pawar, S., Dell, A. I. & Savage, V. M. Dimensionality of consumer search space drives trophic interaction strengths. Nature 486, 485–489 (2012).
Pawar, S., Dell, A. I. & Savage, V. M. in Aquatic Functional Biodiversity: An Ecological and Evolutionary Perspective (eds Belgrano, A., Woodward, G. & Jacob, U.) 3–36 (Academic, Cambridge, 2015).
Carbone, C., Teacher, A. & Rowcliffe, J. M. The costs of carnivory. PLoS Biol. 5, e22 (2007).
Carbone, C., Codron, D., Scofield, C., Clauss, M. & Bielby, J. Geometric factors influencing the diet of vertebrate predators in marine and terrestrial environments. Ecol. Lett. 17, 1553–1559 (2014).
Carbone, C., Mace, G., Roberts, S. & Macdonald, D. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 1997–2000 (1999).
Kiltie, R. A. Scaling of visual acuity with body size. Funct. Ecol. 14, 226–234 (2000).
Field, D. J. Relations between the statistics of natural images and the response properties of cortical cells. J. Opt. Soc. Am. A 4, 2379–2394 (1987).
Wilson, R. P. et al. Turn costs change the value of animal search paths. Ecol. Lett. 16, 1145–1150 (2013).
Blake, R. W. & Domenici, P. Biomechanics in Animal Behaviour (BIOS Scientific, Oxford, 2000).
DeLong, J. P. & Vasseur, D. A. A dynamic explanation of size–density scaling in carnivores. Ecology 93, 470–476 (2012).
Vucic-pestic, O. et al. Allometric functional response model: body masses constrain interaction strengths. J. Anim. Ecol. 79, 249–256 (2010).
De Vries, J. L., Pirk, C. W. W., Bateman, P. W., Cameron, E. Z. & Dalerum, F. Extension of the diet of an extreme foraging specialist, the aardwolf (Proteles cristata). African Zool. 46, 194–196 (2011).
Van Valkenburgh, B. Deja vu: the evolution of feeding morphologies in the Carnivora. Integr. Comp. Biol. 47, 147–163 (2007).
Taylor, C. R., Heglund, N. C. & Maloiy, G. M. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 1–21 (1982).
Pawar, S., Dell, A. I. & Savage, V. M. Pawar et al. reply. Nature 493, E2–E3 (2013).
Dell, A. I., Pawar, S. & Savage, V. M. Temperature dependence of trophic interactions are driven by asymmetry of species responses and foraging strategy. J. Anim. Ecol. 83, 70–84 (2014).
Shipley, L. A., Gross, J. E., Spalinger, D. E., Hobbs, N. T. & Wunder, B. A. The scaling of intake rate in mammalian herbivores. Am. Nat. 143, 1055–1082 (1994).
Ramesh, T., Kalle, R., Sankar, K. & Qureshi, Q. Role of body size in activity budgets of mammals in the Western Ghats of India. J. Trop. Ecol. 31, 315–323 (2015).
Rowcliffe, J. M., Kays, R., Kranstauber, B., Carbone, C. & Jansen, P. A. Quantifying levels of animal activity using camera trap data. Methods Ecol. Evol. 5, 1170–1179 (2014).
Von Buddenbrock, W. Über die kinetische und statische leistung großer und kleiner tiere und ihre bedeutung für den gesamtstoffwechsel. Naturwissenschaften 40, 675–680 (1934).
Huwaldt, J. A. Plot Digitizer (2014); http://plotdigitizer.sourceforge.net
Gittleman, J. L. Carnivore Behavior, Ecology, and Evolution 1st edn (Chapman & Hall, London, 1989).
Tucker, M. A. & Rogers, T. L. Examining predator–prey body size, trophic level and body mass across marine and terrestrial mammals. Proc. R. Soc. B 281, 20142103 (2014).
R Development Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2015).
Felsenstein, J. Phylogenies and the comparative method. Am. Nat 125, 1–15 (1985).
Nyakatura, K. & Bininda-Emonds, O. R. P. Updating the evolutionary history of Carnivora (Mammalia): a new species-level supertree complete with divergence time estimates. BMC Biol. 10, 12–43 (2012).
Harmon, L., Weir, J., Brock, C., Glor, R. & Challenger, W. Geiger: investigating evolutionary radiations. Bioinformatics 24, 129–131 (2008).
Muggeo, V. M. R. segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25 (2008).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-131 (R Foundation for Statistical Computing, Vienna, 2017).
Acknowledgements
We thank V. Muggeo and D.-G. Kontopoulos for advice on the phylogenetically independent contrast and phylogenetic piecewise regression analyses. S.P. was supported by grant NE/M004740/1 awarded by the Natural Environmental Research Council and the Grand Challenges in Ecosystems and the Environment Initiative at Imperial College London.
Author information
Authors and Affiliations
Contributions
M.R., C.C. and S.P. designed the study. S.P. developed the mathematical model. M.R. performed the data compilation and analyses and wrote the first draft of the paper. All authors substantially revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Information, Supplementary Figures 1–10, Supplementary Tables 1–5 and Supplementary References
Rights and permissions
About this article
Cite this article
Rizzuto, M., Carbone, C. & Pawar, S. Foraging constraints reverse the scaling of activity time in carnivores. Nat Ecol Evol 2, 247–253 (2018). https://doi.org/10.1038/s41559-017-0386-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-017-0386-1
This article is cited by
-
Predatory synapsid ecomorphology signals growing dynamism of late Palaeozoic terrestrial ecosystems
Communications Biology (2024)
-
Spatial variance-mass allometry of population density in felids from camera-trapping studies worldwide
Scientific Reports (2020)
-
Logarithmic scales in ecological data presentation may cause misinterpretation
Nature Ecology & Evolution (2018)