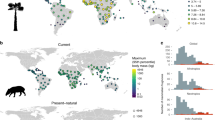
Abstract
Animal-mediated seed dispersal by frugivorous birds and mammals is central to the ecology and functioning of ecosystems, but whether and how frugivory-related traits have affected plant speciation remains little explored. Fruit size is directly linked to plant dispersal capacity and therefore influences gene flow and genetic divergence of plant populations. Using a global species-level phylogeny with comprehensive data on fruit sizes and plant species distributions, we test whether fruit size has affected speciation rates of palms (Arecaceae), a plant family characteristic of tropical rainforests. Globally, the results reveal that palms with small fruit sizes have increased speciation rates compared with those with large (megafaunal) fruits. Speciation of small-fruited palms is particularly high in the understory of tropical rainforests in the New World, and on islands in the Old World. This suggests that frugivory-related traits in combination with geography and the movement behaviour of frugivores can influence the speciation of fleshy-fruited plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kissling, W. D., Böhning–Gaese, K. & Jetz, W. The global distribution of frugivory in birds. Glob. Ecol. Biogeogr. 18, 150–162 (2009).
Fleming, T. H. & Kress, W. J. The Ornaments of Life: Coevolution and Conservation in the Tropics (Chicago Univ. Press, Chicago, 2013).
Nathan, R. & Muller-Landau, H. C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 15, 278–285 (2000).
Givnish, T. J. Ecology of plant speciation. Taxon 59, 1326–1366 (2010).
Kissling, W. D. Has frugivory influenced the macroecology and diversification of a tropical keystone plant family? Res. Ideas Outcomes 3, e14944 (2017).
Lord, J. M. Frugivore gape size and the evolution of fruit size and shape in Southern Hemisphere floras. Austral Ecol. 29, 430–436 (2004).
Jordano, P. in Seeds: The Ecology of Regeneration in Plant Communities 2nd edn (ed. Fenner, M.) 125–166 (CABI, Wallingford, 2000).
Guimarães, P. R. Jr, Galetti, M. & Jordano, P. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE 3, e1745 (2008).
Janzen, D. H. & Martin, P. S. Neotropical anachronisms: the fruits the gomphotheres ate. Science 215, 19–27 (1982).
Haskell, J. P., Ritchie, M. E. & Olff, H. Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature 418, 527–530 (2002).
Milton, K. & May, M. L. Body weight, diet and home range area in primates. Nature 259, 459–462 (1976).
Shanahan, M., Harrison, R. D., Yamuna, R., Boen, W. & Thornton, I. W. B. Colonization of an island volcano, Long Island, Papua New Guinea, and an emergent island, Motmot, in its caldera lake. V. Colonization by figs (Ficus spp.), their dispersers and pollinators. J. Biogeogr. 28, 1365–1377 (2001).
Burney, C. W. & Brumfield, R. T. Ecology predicts levels of genetic differentiation in neotropical birds. Am. Nat. 174, 358–368 (2009).
Salisbury, C. L., Seddon, N., Cooney, C. R. & Tobias, J. A. The latitudinal gradient in dispersal constraints: ecological specialisation drives diversification in tropical birds. Ecol. Lett. 15, 847–855 (2012).
Karr, J. R. Geographical variation in the avifaunas of tropical forest undergrowth. Auk 97, 283–298 (1980).
Smith, J. F. High species diversity in fleshy-fruited tropical understory plants. Am. Nat. 157, 646–653 (2001).
Holbrook, K. M., Smith, T. B. & Hardesty, B. D. Implications of long-distance movements of frugivorous rain forest hornbills. Ecography 25, 745–749 (2002).
Losos, J. B. & Ricklefs, R. E. Adaptation and diversification on islands. Nature 457, 830–836 (2009).
Dransfield, J. et al. Genera Palmarum: The Evolution and Classification of Palms (Kew Publishing, Kew, 2008).
Couvreur, T. L. P., Forest, F. & Baker, W. J. Origin and global diversification patterns of tropical rain forests: inferences from a complete genus-level phylogeny of palms. BMC Biol. 9, 44 (2011).
Zona, S. & Henderson, A. A review of animal-mediated seed dispersal of palms. Selbyana 11, 6–21 (1989).
Faurby, S., Eiserhardt, W. L., Baker, W. J. & Svenning, J.-C. An all-evidence species-level supertree for the palms (Arecaceae). Mol. Phylogenet. Evol. 100, 57–69 (2016).
Brummitt, R. K., Pando, F., Hollis, S. & Brummitt, N. World Geographical Scheme for Recording Plant Distributions (International Working Group on Taxonomic Databases for Plant Sciences (TDWG), Kew, 2001).
FitzJohn, R. G., Maddison, W. P. & Otto, S. P. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Syst. Biol. 58, 595–611 (2009).
FitzJohn, R. G. Diversitree: comparative phylogenetic analyses of diversification in R. Methods Ecol. Evol. 3, 1084–1092 (2012).
Richardson, J. E. & Pennington, R. T. Editorial: Origin of tropical diversity: from clades to communities. Front. Genet. 7, 186 (2016).
Jordano, P., García, C., Godoy, J. A. & García-Castaño, J. L. Differential contribution of frugivores to complex seed dispersal patterns. Proc. Natl Acad. Sci. USA 104, 3278–3282 (2007).
Lenz, J. et al. Seed-dispersal distributions by trumpeter hornbills in fragmented landscapes. Proc. R. Soc. B 278, 2257–2264 (2011).
Galetti, M. et al. Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340, 1086–1090 (2013).
Baker, W. J. & Couvreur, T. L. P. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. II Diversification history and origin of regional assemblages. J. Biogeogr. 40, 286–298 (2013).
Moore, R. P., Robinson, W. D., Lovette, I. J. & Robinson, T. R. Experimental evidence for extreme dispersal limitation in tropical forest birds. Ecol. Lett. 11, 960–968 (2008).
Corlett, R. T. Frugivory and seed dispersal by vertebrates in tropical and subtropical Asia: an update. Glob. Ecol. Conserv. 11, 1–22 (2017).
Fleming, T. H., Breitwisch, R. & Whitesides, G. H. Patterns of tropical vertebrate frugivore diversity. Annu. Rev. Ecol. Syst. 18, 91–109 (1987).
Banin, L. et al. What controls tropical forest architecture? Testing environmental, structural and floristic drivers. Glob. Ecol. Biogeogr. 21, 1179–1190 (2012).
Kissling, W. D. et al. Cenozoic imprints on the phylogenetic structure of palm species assemblages worldwide. Proc. Natl Acad. Sci. USA 109, 7379–7384 (2012).
Baker, W. J. & Couvreur, T. L. P. in Biotic Evolution and Environmental Cchange in Southeast Asia (eds Gower, D. et al.) 164–190 (Cambridge University Press, Cambridge 2012).
Bacon, C. D., Baker, W. J. & Simmons, M. P. Miocene dispersal drives island radiations in the palm tribe Trachycarpeae (Arecaceae). Syst. Biol. 61, 426–442 (2012).
Diamond, J. M., Gilpin, M. E. & Mayr, E. Species-distance relation for birds of the Solomon Archipelago, and the paradox of the great speciators. Proc. Natl Acad. Sci. USA 73, 2160–2164 (1976).
Hughes, C. & Eastwood, R. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl Acad. Sci. USA 103, 10334–10339 (2006).
Kissling, W. D. et al. Quaternary and pre-Quaternary historical legacies in the global distribution of a major tropical plant lineage. Glob. Ecol. Biogeogr. 21, 909–921 (2012).
Morici, C. in Ecologia Insular (eds Fernandez-Palacios, J. M. & Morici, C.) 81–122 (Asociacion Española de Ecología Terrestre, Badajoz, 2004).
Sanín, M. J. et al. The Neogene rise of the tropical Andes facilitated diversification of wax palms (Ceroxylon: Arecaceae) through geographical colonization and climatic niche separation. Bot. J. Linn. Soc. 182, 303–317 (2016).
Svenning, J.-C. On the role of microenvironmental heterogeneity in the ecology and diversification of neotropical rain-forest palms (Arecaceae). Bot. Rev. 67, 1–53 (2001).
Eiserhardt, W. L., Svenning, J.-C., Kissling, W. D. & Balslev, H. Geographical ecology of the palms (Arecaceae): determinants of diversity and distributions across spatial scales. Ann. Bot. 108, 1391–1416 (2011).
Herrera, C. M. Interspecific variation in fruit shape: allometry, phylogeny, and adaptation to dispersal agents. Ecology 73, 1832–1841 (1992).
Lomáscolo, S. B., Levey, D. J., Kimball, R. T., Bolker, B. M. & Alborn, H. T. Dispersers shape fruit diversity in Ficus (Moraceae). Proc. Natl Acad. Sci. USA 107, 14668–14672 (2010).
Voigt, F. A. et al. A comparison of morphological and chemical fruit traits between two sites with different frugivore assemblages. Oecologia 141, 94–104 (2004).
Dominy, N. J., Svenning, J. C. & Li, W. H. Historical contingency in the evolution of primate color vision. J. Hum. Evol. 44, 25–45 (2003).
Ricklefs, R. E. & Renner, S. S. Species richness within families of flowering plants. Evolution 48, 1619–1636 (1994).
Onstein, R. E. & Linder, H. P. Beyond climate: convergence in fast evolving sclerophylls in Cape and Australian Rhamnaceae predates the Mediterranean climate. J. Ecol. 104, 665–677 (2016).
Baker, W. J. et al. Complete generic-level phylogenetic analyses of palms (Arecaceae) with comparisons of supertree and supermatrix approaches. Syst. Biol. 58, 240–256 (2009).
Govaerts, R. & Dransfield, J. World Checklist of Palms (Royal Botanic Gardens Kew, 2005).
Kreft, H., Jetz, W., Mutke, J., Kier, G. & Barthlott, W. Global diversity of island floras from a macroecological perspective. Ecol. Lett. 11, 116–127 (2008).
Weigelt, P., Jetz, W. & Kreft, H. Bioclimatic and physical characterization of the world’s islands. Proc. Natl Acad. Sci. USA 110, 15307–15312 (2013).
Maddison, W. P., Midford, P. E. & Otto, S. P. Estimating a binary character’s effect on speciation and extinction. Syst. Biol. 56, 701–710 (2007).
Rabosky, D. L. & Goldberg, E. E. Model inadequacy and mistaken inferences of trait-dependent speciation. Syst. Biol. 64, 340–355 (2015).
Maddison, W. P. & FitzJohn, R. G. The unsolved challenge to phylogenetic correlation tests for categorical characters. Syst. Biol. 64, 127–136 (2015).
Davis, M. P., Midford, P. E. & Maddison, W. Exploring power and parameter estimation of the BiSSE method for analyzing species diversification. BMC Evol. Biol. 13, 1–11 (2013).
R Development Core Team R: A Language and Environment for Statistical Computing, Version 3.1.0 (R Foundation for Statistical Computing, Vienna, 2014); http://www.R-project.org
Bruggeman, J., Heringa, J. & Brandt, B. W. PhyloPars: estimation of missing parameter values using phylogeny. Nucleic Acids Res. 37, W179–W184 (2009).
Baker, W. J. & Couvreur, T. L. P. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. J. Biogeogr. 40, 274–285 (2013).
FitzJohn, R. G. Quantitative traits and diversification. Syst. Biol. 59, 619–633 (2010).
Rabosky, D. L. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS ONE 9, e89543 (2014).
Rabosky, D. L. & Huang, H. A robust semi-parametric test for detecting trait-dependent diversification. Syst. Biol. 65, 181–193 (2016).
Acknowledgements
We thank H. Balslev, A. Barfod, A. Blach-Overgaard, F. Borchsenius, J. Dransfield, W. Eiserhardt and M. J. Sanín for discussions about palm biology and J. Dransfield,A. Barfod and A. J. Henderson for the use of pictures for Fig. 1. We thank J. Ollerton for constructive comments on an earlier version of the manuscript. W.D.K. was supported by the University of Amsterdam (starting grant), the Danish Council for Independent Research–Natural Sciences (grant 11-106163) and the Netherlands Organisation for Scientific Research (grant 824.15.007). W.J.B. was supported by a grant from the Garfield Weston Foundation to the Global Tree Seed Bank Project at the Royal Botanic Gardens, Kew. J.C.S. was supported by the European Research Council (ERC-2012-StG-310886-HISTFUNC), and also considers this work a contribution to his VILLUM Investigator project 'Biodiversity Dynamics in a Changing World' funded by VILLUM FONDEN.
Author information
Authors and Affiliations
Contributions
W.D.K. conceived the idea; W.D.K. and R.E.O. designed the study; W.D.K. and R.E.O. collected data; R.E.O. analysed the data; R.E.O. and W.D.K. wrote the manuscript; all authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary notes, figures, tables, data sources and references
Supplementary Table 1
Summary statistics of fruit sizes for each palm genus
Rights and permissions
About this article
Cite this article
Onstein, R.E., Baker, W.J., Couvreur, T.L.P. et al. Frugivory-related traits promote speciation of tropical palms. Nat Ecol Evol 1, 1903–1911 (2017). https://doi.org/10.1038/s41559-017-0348-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-017-0348-7
This article is cited by
-
Divergence in functional traits in seven species of neotropical palms of different forest strata
Oecologia (2023)
-
Uneven patterns of palm species loss due to climate change are not driven by their sexual systems
Biodiversity and Conservation (2023)
-
Climate change impacts on the Copernicia alba and Copernicia prunifera (Arecaceae) distribution in South America
Brazilian Journal of Botany (2022)
-
Global and regional ecological boundaries explain abrupt spatial discontinuities in avian frugivory interactions
Nature Communications (2022)
-
Mycorrhizal types influence island biogeography of plants
Communications Biology (2021)