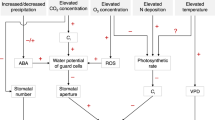
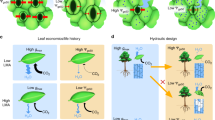
Abstract
Stomatal pores on leaf surfaces respond to environmental and physiological signals to regulate leaf gas exchange. Mathematical models can predict stomatal conductance (g s), with one parameter (m or g l) reflecting the sensitivity of g s to the photosynthetic rate (A), atmospheric carbon dioxide concentration and atmospheric humidity, and a second parameter (g 0) representing the minimum g s. Such models are solved iteratively with a photosynthesis model to form the core of many models of crop or ecosystem carbon and water fluxes. For three decades, g s models have frequently been used assuming fixed parameter values for m or g 1 and g 0 across species and major plant functional types. This study of temperate tree species reveals significant interspecific variation in stomatal function. Applying species-specific parameterizations substantially reduced error in model predictions of g s by 34 to 64% and A by 52 to 60% and resulted in significant correlation between modelled and measured values. This work challenges the long-held assumption of fixed parameter values and, in doing so, suggests an approach for reducing modelling error across a wide range of ecological and agricultural applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 (2003).
Berry, J. A., Beerling, D. J. & Franks, P. J. Stomata: key players in the Earth system, past and present. Curr. Opin. Plant Biol. 13, 233–240 (2010).
Ball, J., Woodrow, I. & Berry J. in Progress in Photosynthesis Research (ed. Biggens, J.) 221–224 (Martinus Nijhoff, Dordrecht, 1987).
Medlyn, B. E. et al. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Global Change Biol. 17, 2134–2144 (2011).
Leuning, R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ. 18, 339–355 (1995).
Farquhar, G. D., Caemmerer, S. V. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Collatz, G. J., Ball, J. T., Grivet, C. & Berry, J. A. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration—a model that includes a laminar boundary layer. Agr. Forest Meteorol. 54, 107–136 (1991).
Foley, J. A. et al. An integrated biosphere model of land surface processes, terrestrial carbon balance, and vegetation dynamics. Global Biogeochem. Cycles 10, 603–628 (1996).
Sellers, P. J. et al. A revised land surface parameterization (SiB2) for atmospheric GCMs. 1. Model formulation. J. Clim. 9, 676–705 (1996).
Bounoua, L. et al. Interactions between vegetation and climate: radiative and physiological effects of doubled atmospheric CO2. J. Clim. 12, 309–324 (1999).
Moorcroft, P. R., Hurtt, G. C. & Pacala, S. W. A method for scaling vegetation dynamics: the ecosystem demography model (ED). Ecol. Monogr. 71, 557–585 (2001).
Twine, T. E. et al. Impacts of elevated CO2 concentration on the productivity and surface energy budget of the soybean and maize agroecosystem in the Midwest USA. Global Change Biol. 19, 2838–2852 (2013).
Somerville, C., Youngs, H., Taylor, C., Davis, S. C. & Long, S. P. Feedstocks for lignocellulosic biofuels. Science 329, 790–792 (2010).
Lobell, D. B., Sibley, A. & Ortiz-Monasterio, J. I. Extreme heat effects on wheat senescence in India. Nat. Clim. Change 2, 186–189 (2012).
Cox, P. M. et al. Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature 494, 341–344 (2013).
Kowalczyk, E. A. et al. The CSIRO Atmosphere Biosphere Land Exchange (CABLE) Model for use in Climate Models and as an Offline Model (CSIRO Marine and Atmospheric Research, 2006).
Oleson, K. W. et al. Technical Description of Version 4.0 of the Community Land Model (CLM) (National Center for Atmospheric Research, 2010).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Givnish, T. in On the Economy of Plant Form and Function (ed. Givnish, T. J.) 171–213 (Cambridge Univ. Press, Cambridge, 1986).
De Kauwe, M. G. et al. Forest water use and water use efficiency at elevated CO2: a model−data intercomparison at two contrasting temperate forest FACE sites. Global Change Biol. 19, 1759–1779 (2013).
Smith, N. G. & Dukes, J. S. Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Global Change Biol. 19, 45–63 (2013).
Lin, Y.-S. et al. Optimal stomatal behaviour around the world. Nat. Clim. Change 5, 459–464 (2015).
Kattge, J. et al. TRY—a global database of plant traits. Global Change Biol. 17, 2905–2935 (2011).
Wilson, B. T., Lister, A. J., Riemann, R. I. & Griffith, D. M. Live Tree Species Basal Area of the Contiguous United States (2000–2009) http://dx.doi.org/10.2737/RDS-2013-0013 (USDA Forest Service, Newtown Square, PA, 2013).
Scheiter, S., Langan, L. & Higgins, S. I. Next-generation dynamic global vegetation models: learning from community ecology. New Phytol. 198, 957–969 (2013).
Wang, D., LeBauer, D. & Dietze, M. Predicting yields of short-rotation hybrid poplar (Populus spp.) for the United States through model-data synthesis. Ecol. Appl. 23, 944–958 (2013).
Lebauer, D. S., Wang, D., Richter, K. T., Davidson, C. C. & Dietze, M. C. Facilitating feedbacks between field measurements and ecosystem models. Ecol. Monogr. 83, 133–154 (2013).
Cernusak, L. A. et al. Stable isotopes in leaf water of terrestrial plants. Plant Cell Environ. 39, 1087–1102 (2016).
Aranibar, J. N. et al. Combining meteorology, eddy fluxes, isotope measurements, and modeling to understand environmental controls of carbon isotope discrimination at the canopy scale. Global Change Biol. 12, 710–730 (2006).
Diefendorf, A. F., Mueller, K. E., Wing, S. L., Koch, P. L. & Freeman, K. H. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl Acad. Sci. USA 107, 5738–5743 (2010).
Suits, N. S. et al. Simulation of carbon isotope discrimination of the terrestrial biosphere. Global Biogeochem. Cycles 19, GB1017 (2005).
Jarvis, P. G. & McNaughton, K. G. Stomatal control of transpiration—scaling up from leaf to region. Adv. Ecol. Res. 15, 1–49 (1986).
Dietze, M. C. et al. A quantitative assessment of a terrestrial biosphere model’s data needs across North American biomes. J. Geophys. Res. Biogeosci. 119, 286–300 (2014).
Bernacchi, C. J., Kimball, B. A., Quarles, D. R., Long, S. P. & Ort, D. R. Decreases in stomatal conductance of soybean under open-air elevation of CO2 are closely coupled with decreases in ecosystem evapotranspiration. Plant Phys. 143, 134–144 (2007).
De Kauwe, M. G. et al. A test of an optimal stomatal conductance scheme within the CABLE land surface model. Geosci. Model Dev. 8, 431–452 (2015).
Cox, P. M. et al. The impact of new land surface physics on the GCM simulation of climate and climate sensitivity. Clim. Dynam. 15, 183–203 (1999).
Medvigy, D., Wofsy, S. C., Munger, J. W., Hollinger, D. Y. & Moorcroft, P. R. Mechanistic scaling of ecosystem function and dynamics in space and time: Ecosystem Demography model version 2. J. Geophys. Res. Biogeosci. 114, G01002 (2009).
Kerstiens, G. Cuticular water permeability and its physiological significance. J. Exp. Bot. 47, 1813–1832 (1996).
Snyder, K. A., Richards, J. H. & Donovan, L. A. Night-time conductance in C3 and C4 species: do plants lose water at night? J. Exp. Bot. 54, 861–865 (2003).
Barnard, D. M. & Bauerle, W. L. The implications of minimum stomatal conductance on modeling water flux in forest canopies. J. Geophys. Res. Biogeosci. 118, 1322–1333 (2013).
Condon, A. G., Richards, R. A., Rebetzke, G. J. & Farquhar, G. D. Breeding for high water-use efficiency. J. Exp. Bot. 55, 2447–2460 (2004).
Oren, R. et al. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 22, 1515–1526 (1999).
Bonal, D., Sabatier, D., Montpied, P., Tremeaux, D. & Guehl, J. M. Interspecific variability of delta δ13C among trees in rainforests of French Guiana: functional groups and canopy integration. Oecologia 124, 454–468 (2000).
Brodribb, T. J. & McAdam, S. A. M. Passive origins of stomatal control in vascular plants. Science 331, 582–585 (2011).
Wullschleger, S. D. Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/C i curves from 109 species. J. Exp. Bot. 44, 907–920 (1993).
Leakey, A. D. B., Bernacchi, C. J., Ort, D. R. & Long, S. P. Long-term growth of soybean at elevated CO2 does not cause acclimation of stomatal conductance under fully open-air conditions. Plant Cell Environ. 29, 1794–1800 (2006).
Ainsworth, E. A. et al. Does elevated atmospheric CO2 alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? J. Exp. Bot. 58, 579–591 (2007).
Long, S. P. & Bernacchi, C. J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 54, 2393–2401 (2003).
Campbell, G. S. & Norman, J. M. An Introduction to Environmental Biophysics 2nd edn (Springer Science + Business Media, New York, 1998).
Powell, M. J. D. in Numerical Methods for Nonlinear Algebraic Equations (ed. Rabinowitz, P.) Ch. 7 (Gordon and Breach Science, London, 1970).
Bernacchi, C. J., Singsaas, E. L., Pimentel, C., Portis, A. R. & Long, S. P. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 24, 253–259 (2001).
Bernacchi, C. J., Pimentel, C. & Long, S. P. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ. 26, 1419–1430 (2003).
Acknowledgements
Support for this project was provided by the Illinois Biomathematics Program via a grant from National Science Foundation (Division of Undergraduate Education, Award # 1129198) and the Energy Biosciences Institute. The authors thank G. Kling and the Energy Biosciences Institute Bioenergy Farm for access to the plant material used in this study, M. Dietze for assistance with model assembly, D. Rosenthal for technical support with photosynthetic gas exchange, E. Ainsworth for comments on the draft manuscript and M. de Kauwe for valuable reviews of the original manuscript.
Author information
Authors and Affiliations
Contributions
A.D.B.L. and K.J.W. designed the experiment. K.J.W., T.M.W., M.A. and D.W. collected the data. K.J.W. performed the modelling. K.J.W., T.M.W. and A.D.B.L. performed the statistical analyses. A.D.B.L. and K.J.W. wrote the paper with significant revisions from T.M.W. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Tables 1–3; Supplementary Figures 1–10
Supplementary Data 1
In situ gas exchange data
Supplementary Data 2
Lab gas exchange data
Supplementary Data 3
In situ model fits
Supplementary Data 4
Model inputs and outputs
Supplementary Data 5
Coupled model code
Rights and permissions
About this article
Cite this article
Wolz, K.J., Wertin, T.M., Abordo, M. et al. Diversity in stomatal function is integral to modelling plant carbon and water fluxes. Nat Ecol Evol 1, 1292–1298 (2017). https://doi.org/10.1038/s41559-017-0238-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-017-0238-z
This article is cited by
-
Compound heat and moisture extreme impacts on global crop yields under climate change
Nature Reviews Earth & Environment (2022)
-
Transcriptome profiling reveals key genes in regulation of the tepal trichome development in Lilium pumilum D.C.
Plant Cell Reports (2021)
-
Quantifying impacts of enhancing photosynthesis on crop yield
Nature Plants (2019)
-
Predicting light-induced stomatal movements based on the redox state of plastoquinone: theory and validation
Photosynthesis Research (2019)
-
Detecting the fingerprint of drought across Europe’s forests: do carbon isotope ratios and stem growth rates tell similar stories?
Forest Ecosystems (2017)