Abstract
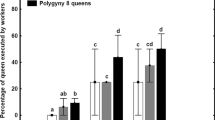
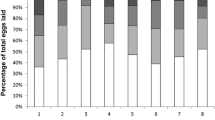
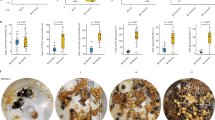
Social insect queen pheromones can be interpreted as the queen’s means of sterilizing her workers, or as an honest signal of queen presence that benefits both parties. Co-mapping worker reproductive behaviour and queen cuticular hydrocarbon (CH) composition and quantity on a phylogeny of 21 stingless bee species showed that there are no associations between these traits. Furthermore, three species that have independently evolved facultative worker sterility are unexceptional in their queen CH. Combined, our analysis suggests that the action of stingless bee queen CHs are best interpreted as a signal of queen presence and not as a chemical contraceptive.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nunes, T. M. et al. Sci. Rep. 4, 7449 (2014).
van Oystaeyen, A. et al. Science 343, 287–290 (2014).
Peso, M., Elgar, M. A. & Barron, A. B. Biol. Rev. 90, 542–559 (2015).
Grüter, C. & Keller, L. Curr. Opin. Neurobiol. 38, 6–11 (2016).
Oi, C. A. et al. Bioessays 37, 808–821 (2015).
Strauss, K. et al. Behav. Ecol. Sociobiol. 16, 1523–1531 (2008).
Keller, L. & Nonacs, P. Anim. Behav. 45, 787–794 (1993).
Ronai, I., Vergoz, V. & Oldroyd, B. P. Adv. Stud. Behav. 48, 251–317 (2016).
Kerr, W. E. & Maule, V. J. New York Entomol. Soc. 72, 2–18 (1964).
Palmer, K. A., Oldroyd, B. P., Quezada-Euán, J. J. V., Paxton, R. J. & May-Itza, W. D. Mol. Ecol. 11, 2107–2113 (2002).
Ratnieks, F. L. W. Am. Nat. 132, 217–236 (1988).
Hammond, R. L. & Keller, L. PLoS Biol. 2, e248 (2004).
Wenseleers, T., Helanterä, H., Alves, D. A., Dueñez-Guzmán, E. & Pamilo, P. Biol. Lett. 9, 20130334 (2013).
Tóth, E., Queller, D. C., Dollin, A. & Strassmann, J. E. Insect. Soc. 51, 1–11 (2004).
Rasmussen, C. & Cameron, S. A. Biol. J. Linn. Soc. 99, 206–232 (2010).
Harvey, P. H . & Pagel, M. D. The Comparative Method in Evolutionary Biology (Oxford Univ. Press, 1991).
Nunes, T. M., Turatti, I. C. C., Lopes, N. P. & Zucchi, R. J. Chem. Ecol. 35, 1172–1180 (2009).
Ives, A. R. & Garland, T. J. Syst. Biol. 59, 9–26 (2010).
Ho, L. S. T. & Ané, C. Syst. Biol. 63, 397–408 (2014).
Burnham, K. P . & Anderson, D. R. Model Selection and Multimodel Inference 2nd edn (Springer, 2002).
Acknowledgements
The authors are grateful to E.A.B. Almeida (FFCLRP - Universidade de São Paulo) for his important contributions regarding the interpreting and coding of characters, and the analysis of phylogenetic comparative data, which were crucial to the initial development of this study. The authors are also thankful to T. Heard for providing Australian stingless bees queens. This research was supported by FAPESP (L.G.E. 2013/ 01918-1; T.M.N. 2013/09263-4, N.P.L. 2014/50265-3) and CNPq (T.M.N. 165823/2015-1).
Author information
Authors and Affiliations
Contributions
T.M.N., L.G.E. and N.P.L. designed and conceived the study. T.M.N. and S.M. collected the biological material and performed behavioural analysis on worker oviposition. T.M.N., I.C.T. and N.P.L. performed gas chromatography mass spectrometry analyses. T.M.N. and L.G.E. performed phylogenetic and statistical analyses. B.P.O. determined the conceptual framework for the paper, and T.M.N., L.G.E. and B.P.O. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Tables 1–5, two Supplementary Figures and Supplementary References. (PDF 623 kb)
Supplementary Code
Supporting data and R code. (ZIP 11 kb)
Rights and permissions
About this article
Cite this article
Nunes, T., Oldroyd, B., Elias, L. et al. Evolution of queen cuticular hydrocarbons and worker reproduction in stingless bees. Nat Ecol Evol 1, 0185 (2017). https://doi.org/10.1038/s41559-017-0185
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-017-0185
This article is cited by
-
Queen loss changes behavior and increases longevity in a stingless bee
Behavioral Ecology and Sociobiology (2020)
-
Preface: Pheromone-Mediation of Female Reproduction and Reproductive Dominance in Social Species
Journal of Chemical Ecology (2018)