Abstract
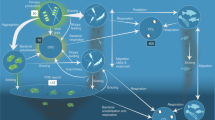
Antarctic krill (Euphausia superba)—one of the most abundant animal species on Earth—exhibits a five to six year population cycle, with oscillations in biomass exceeding one order of magnitude. Previous studies have postulated that the krill cycle is induced by periodic climatological factors, but these postulated drivers neither show consistent agreement, nor are they supported by quantitative models. Here, using data analysis complemented with modelling of krill ontogeny and population dynamics, we identify intraspecific competition for food as the main driver of the krill cycle, while external climatological factors possibly modulate its phase and synchronization over large scales. Our model indicates that the cycle amplitude increases with reduction of krill loss rates. Thus, a decline of apex predators is likely to increase the oscillation amplitude, potentially destabilizing the marine food web, with drastic consequences for the entire Antarctic ecosystem.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hewitt, R. P., Demer, D. A. & Emery, J. H. An 8-year cycle in krill biomass density inferred from acoustic surveys conducted in the vicinity of the South Shetland Islands during the austral summers of 1991–1992 through 2001–2002. Aquat. Living Resour. 16, 205–213 (2003).
Quetin, L. B. & Ross, R. M. Episodic recruitment in Antarctic krill Euphausia superba in the Palmer LTER study region. Mar. Ecol. Prog. Ser. 259, 185–200 (2003).
Fielding, S. et al. Interannual variability in Antarctic krill (Euphausia superba) density at South Georgia, Southern Ocean: 1997–2013. ICES J. Mar. Sci. 71, 2578–2588 (2014).
Ross, R. M. et al. Trends, cycles, interannual variability for three pelagic species west of the Antarctic Peninsula 1993–2008. Mar. Ecol. Prog. Ser. 515, 11–32 (2014).
Atkinson, A. et al. Oceanic circumpolar habitats of Antarctic krill. Mar. Ecol. Prog. Ser. 362, 1–23 (2008).
Atkinson, A., Siegel, V., Pakhomov, E. & Rothery, P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432, 100–103 (2004).
Knox, G. A. Biology of the Southern Ocean 2nd edn (CRC, 2006).
Atkinson, A., Siegel, V., Pakhomov, E. A., Jessopp, M. J. & Loeb, V. A re-appraisal of the total biomass and annual production of Antarctic krill. Deep-Sea Res. I 56, 727–740 (2009).
Saba, G. K. et al. Winter and spring controls on the summer food web of the coastal West Antarctic Peninsula. Nat. Commun. 5, 4318 (2014).
Ducklow, H. W. et al. Marine pelagic ecosystems: the West Antarctic Peninsula. Phil. Trans. R. Soc. B 362, 67–94 (2007).
Loeb, V. in The Impact of Environmental Variability on Ecological Systems (eds Vasseur, D. A. & McCann, K. S. ) 197–225 (Springer, 2007).
Steinberg, D. K. et al. Long-term (1993–2013) changes in macrozooplankton off the Western Antarctic Peninsula. Deep-Sea Res. I 101, 54–70 (2015).
Quetin, L. B., Ross, R. M., Fritsen, C. H. & Vernet, M. Ecological responses of Antarctic krill to environmental variability: can we predict the future? Antarct. Sci. 19, 253–266 (2007).
Nicol, S. Krill, currents, and sea ice: Euphausia superba and its changing environment. BioScience 56, 111–120 (2006).
Ross, R. M., Quetin, L. B. & Kirsch, E. Effect of temperature on developmental times and survival of early larval stages of Euphausia superba Dana. J. Exp. Mar. Biol. Ecol. 121, 55–71 (1988).
Meyer, B. & Oettl, B. Effects of short-term starvation on composition metabolism of larval Antarctic krill, Euphausia superba. Mar. Ecol. Prog. Ser. 292, 263–270 (2005).
Ikeda, T. & Dixon, P. Body shrinkage as a possible over-wintering mechanism of the Antarctic krill, Euphausia superba Dana. J. Exp. Mar. Biol. Ecol. 62, 143–151 (1982).
Ross, R. M., Quetin, L. B. & Haberman, K. L. Interannual and seasonal variability in short-term grazing impact of Euphausia superba in nearshore and offshore waters west of the Antarctic Peninsula. J. Mar. Syst. 17, 261–273 (1998).
Moran, P. A. P. The statistical analysis of the Canadian lynx cycle. Aust. J. Zool. 1, 291–298 (1953).
Massie, T. M., Weithoff, G., Kuckländer, N., Gaedke, U. & Blasius, B. Enhanced Moran effect by spatial variation in environmental autocorrelation. Nat. Commun. 6, 5993 (2015).
Smith, L. V. et al. Preliminary investigation into the stimulation of phytoplankton photophysiology and growth by whale faeces. J. Exp. Mar. Biol. Ecol. 446, 1–9 (2013).
Murphy, E. J. et al. Spatial and temporal operation of the Scotia Sea ecosystem: a review of large-scale links in a krill centred food web. Phil. Trans. R. Soc. B 362, 113–148 (2007).
Reid, K. & Croxall, J. P. Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proc. R. Soc. Lond. B 268, 377–384 (2001).
Abrams, P. A., Brassil, C. E. & Holt, R. D. Dynamics and responses to mortality rates of competing predators undergoing predator–prey cycles. Theor. Popul. Biol. 64, 163–176 (2003).
Fraser, W. R. & Hofmann, E. E. A predator’s perspective on causal links between climate change, physical forcing and ecosystem. Mar. Ecol. Prog. Ser. 265, 1–15 (2003).
Pennington, M. Efficient estimators of abundance, for fish and plankton surveys. Biometrics 39, 281–286 (1983).
Atkinson, A. et al. Fitting Euphausia superba into Southern Ocean food-web models: a review of data sources and their limitations. CCAMLR Sci. 19, 219–245 (2012).
Pakhomov, E. A. Demographic studies of Antarctic krill Euphausia superba in the Cooperation and Cosmonaut seas(Indian sector of the Southern Ocean). Mar. Ecol. Prog. Ser. 119, 45–61 (1995).
Meyer, B. The overwintering of Antarctic krill, Euphausia superba, from an ecophysiological perspective. Polar Biol. 35, 15–37 (2012).
de Roos, A. M & Persson, L. Population and Community Ecology of Ontogenetic Development (Princeton Univ. Press, 2013).
De Roos, A. M., Diekmann, O. & Metz, J. A. J. Studying the dynamics of structured population models: a versatile technique and its application to Daphnia. Am. Nat. 139, 123–147 (1992).
Quetin, L. B. & Ross, R. M. Depth distribution of developing Euphausia superba embryos, predicted from sinking rates. Mar. Biol. 79, 47–53 (1984).
Atkinson, A. et al. Natural growth rates in Antarctic krill (Euphausia superba): II. Predictive models based on food, temperature, body length, sex, and maturity stage. Limnol. Oceanogr. 51, 973–987 (2006).
Meyer, B. et al. Physiology, growth and development of larval krill Euphausia superba in autumn and winter in the Lazarev Sea, Antarctica. Limnol. Oceanogr. 54, 1595–1614 (2009).
Lowe, A. T., Ross, R. M., Quetin, L. B., Vernet, M. & Fritsen, C. H. Simulating larval Antarctic krill growth and condition factor during fall and winter in response to environmental variability. Mar. Ecol. Prog. Ser. 452, 27–43 (2012).
Harrington, S. A. & Ikeda, T. Laboratory observations on spawning, brood size and egg hatchability of the Antarctic krill Euphausia superba from Prydz Bay, Antarctica. Mar. Biol. 92, 231–235 (1986).
Siegel, V. & Loeb, V. Length and age at maturity of Antarctic krill. Antarct. Sci. 6, 479–482 (1994).
Atkinson, A., Meyer, B., Bathmann, U., Hagen, W. & Schmidt, K. Feeding and energy budget of Antarctic krill Euphausia superba at the onset of winter: II. Juveniles and adults. Limnol. Oceanogr. 47, 953–966 (2002).
Meyer, B. et al. Seasonal variation in body composition, metabolic activity, feeding, and growth of adult krill Euphausia superba in the Lazarev Sea. Mar. Ecol. Prog. Ser. 398, 1–18 (2010).
Ross, R. M., Quetin, L. B., Baker, K. S., Vernet, M. & Smith, R. C. Growth limitation in young Euphausia superba under field conditions. Limnol. Oceanogr. 45, 31–43 (2000).
Englund, G. & Leonardsson, K. Scaling up the functional response for spatially heterogeneous systems. Ecol. Lett. 11, 440–449 (2008).
Hofmann, E. E. & Lascara, C. M. Modeling the growth dynamics of Antarctic krill Euphausia superba. Mar. Ecol. Prog. Ser. 194, 219–231 (2000).
Gentleman, W., Leising, A., Frost, B., Strom, S. & Murray, J. Functional responses for zooplankton feeding on multiple resources: a review of assumptions and biological dynamics. Deep-Sea Res. II. 50, 2847–2876 (2003).
Ryabov, A. B., Morozov, A. & Blasius, B. Imperfect prey selectivity of predators promotes biodiversity and irregularity in food webs. Ecol. Lett. 18, 1262–1269 (2015).
Sailley, S. F. et al. Carbon fluxes and pelagic ecosystem dynamics near two Western Antarctic Peninsula Adélie penguin colonies: an inverse model approach. Mar. Ecol. Prog. Ser. 492, 253–272 (2013).
Kawaguchi, S., Candy, S. G., King, R., Naganobu, M. & Nicol, S. Modelling growth of Antarctic krill. I. Growth trends with sex, length, season, and region. Mar. Ecol. Prog. Ser. 306, 1–15 (2006).
Tarling, G. A. et al. Recruitment of Antarctic krill Euphausia superba in the South Georgia region: adult fecundity and the fate of larvae. Mar. Ecol. Prog. Ser. 331, 161–179 (2007).
Pakhomov, E. A. Natural age-dependent mortality rates of Antarctic krill Euphausia superba Dana in the Indian sector of the Southern Ocean. Polar Biol. 15, 69–71 (1995).
Brinton, E. & Townsend, A. W. Regional relationships between development and growth in larvae of Antarctic krill, Euphausia superba, from field samples. J. Crustacean Biol. 4, 224–246 (1984).
Behrenfeld, M. J. Abandoning Sverdrup’s critical depth hypothesis on phytoplankton blooms. Ecology 91, 977–989 (2010).
Acknowledgements
We thank A. Atkinson, L. B. Quetin and R. M. Ross for useful comments on the manuscript; A.B.R. acknowledges support from the Helmholtz Virtual Institute PolarTime (VH-VI-500); A.M.d.R. is supported by funding from the European Research Council under the European Unions Seventh Framework Programme (FP/2007–2013)/ERC Grant Agreement No. 322814. This work contributes to the PACES (Polar Regions and Coasts in a changing Earth System) program (Topic 1, WP 5) of the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research. Data on krill abundance and biomass were retrieved from the Palmer LTER data archive (http://pal.lternet.edu/data) and data on size distributions were provided by L. B. Quetin and R. M. Ross. The Palmer LTER research was supported by the National Science Foundation, Office of Polar Programs, under Award Nos. OPP-9011927, OPP-9632763, OPP-0217282, ANT-1010688 and PLR-1440435, the Regents of the University of California, the University of California at Santa Barbara, and the Marine Science Institute, UCSB.
Author information
Authors and Affiliations
Contributions
All authors designed the research; A.B.R., A.M.d.R., B.B. developed the model; A.B.R., S.K., B.M. parametrized the model, A.B.R. performed computer experiments and data analysis; A.B.R. and B.B. with contributions from other authors wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–12, Supplementary Tables 1–5 (PDF 1500 kb)
Supplementary Code
C++ source code of the ontogenetic krill model. The archive includes computer model C++ code and additional files, which specify the initial conditions, model parameters, and settings tailoring the model output. All files should be copied into the same folder and compiled with gcc or a similar C++ compiler. The parameters of krill and resource dynamical model are specified in params.ini, the parameters of the simulation output, initial conditions, etc. are specified in the files named “krill.*”. To solve the differential equations, we use the Escalator Boxcar Train approach, see https://staff.fnwi.uva.nl/a.m.deroos/EBT/ for a detailed description of the solver setup. Any additional information can be requested from the corresponding author. (ZIP 94 kb)
Rights and permissions
About this article
Cite this article
Ryabov, A., de Roos, A., Meyer, B. et al. Competition-induced starvation drives large-scale population cycles in Antarctic krill. Nat Ecol Evol 1, 0177 (2017). https://doi.org/10.1038/s41559-017-0177
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-017-0177
This article is cited by
-
The impact of salps (Salpa thompsoni) on the Antarctic krill population (Euphausia superba): an individual-based modelling study
Ecological Processes (2023)
-
Climate change impacts on Antarctic krill behaviour and population dynamics
Nature Reviews Earth & Environment (2023)
-
Estimating the average distribution of Antarctic krill Euphausia superba at the northern Antarctic Peninsula during austral summer and winter
Polar Biology (2022)
-
Improved Properties of Cellulose/Antarctic Krill Protein Composite Fibers with a Multiple Cross-Linking Network
Advanced Fiber Materials (2022)
-
An alternating active-dormitive strategy enables disadvantaged prey to outcompete the perennially active prey through Parrondo’s paradox
BMC Biology (2021)