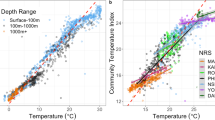
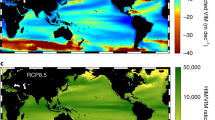
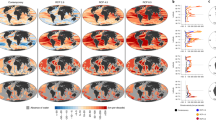
Abstract
Temperature drives global ocean patterns of biodiversity, shaping thermal niches through thresholds of thermal tolerance. Global warming is predicted to change thermal range bounds, yet research has primarily focused on temperature at the sea surface, while knowledge of changes through the depths of the water column is lacking. Here, using daily observations from ocean sites and model simulations, we track shifts in ocean temperatures, focusing on the emergence of thermal ranges whose future lower bounds exceed current upper bounds. These emerge below 50 m depth as early as ~2040 with high anthropogenic emissions, yet are delayed several decades for reduced emission scenarios. By 2100, concomitant changes in both lower and upper boundaries can expose pelagic ecosystems to thermal environments never experienced before. These results suggest the redistribution of marine species might differ across depth, highlighting a much more complex picture of the impact of climate change on marine ecosystems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Interpolated data presented in the paper can be accessed via Zenodo at https://doi.org/10.5281/zenodo.6940283.
Code availability
All code used in the current study is available from the corresponding author upon reasonable request.
References
Barnett, T. P. et al. Penetration of human-induced warming into the world’s oceans. Science 309, 284–287 (2005).
Levitus, S. et al. Global ocean heat content 1955–2008 in light of recently revealed instrumentation problems. Geophys. Res. Lett. 36, L07608 (2009).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925 (2013).
García Molinos, J. et al. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change 6, 83–88 (2016).
Free, C. M. et al. Impacts of historical warming on marine fisheries production. Science 363, 979–983 (2019).
Hughes, N. F. & Grand, T. C. Physiological ecology meets the ideal-free distribution: predicting the distribution of size-structured fish populations across temperature gradients. Environ. Biol. Fishes 59, 285–298 (2000).
Tittensor, D. P. et al. Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101 (2010).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830 (2011).
Waldock, C., Stuart‐Smith, R. D., Edgar, G. J., Bird, T. J. & Bates, A. E. The shape of abundance distributions across temperature gradients in reef fishes. Ecol. Lett. 22, 685–696 (2019).
Stuart-Smith, R. D., Edgar, G. J. & Bates, A. E. Thermal limits to the geographic distributions of shallow-water marine species. Nat. Ecol. Evol. 1, 1846–1852 (2017).
Pinsky, M. L., Worm, B., Fogarty, M. J., Sarmiento, J. L. & Levin, S. A. Marine taxa track local climate velocities. Science 341, 1239–1242 (2013).
Beaugrand, G., Edwards, M., Raybaud, V., Goberville, E. & Kirby, R. R. Future vulnerability of marine biodiversity compared with contemporary and past changes. Nat. Clim. Change 5, 695–701 (2015).
Trisos, C. H., Merow, C. & Pigot, A. L. The projected timing of abrupt ecological disruption from climate change. Nature 580, 496–501 (2020).
Levin, L. A. & Le Bris, N. The deep ocean under climate change. Science 350, 766–768 (2015).
Deutsch, C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (2008).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690 (2012).
Radeloff, V. C. et al. The rise of novelty in ecosystems. Ecol. Appl. 25, 2051–2068 (2015).
Lotterhos, K. E., Láruson, Á. J. & Jiang, L.-Q. Novel and disappearing climates in the global surface ocean from 1800 to 2100. Sci. Rep. 11, 15535 (2021).
Mora, C. et al. The projected timing of climate departure from recent variability. Nature 502, 183–187 (2013).
Henson, S. A. et al. Rapid emergence of climate change in environmental drivers of marine ecosystems. Nat. Commun. 8, 14682 (2017).
Séférian, R. et al. Evaluation of CNRM Earth System Model, CNRM‐ESM2‐1: role of Earth system processes in present‐day and future climate. J. Adv. Model. Earth Syst. 11, 4182–4227 (2019).
Gidden, M. J. et al. Global emissions pathways under different socioeconomic scenarios for use in CMIP6: a dataset of harmonized emissions trajectories through the end of the century. Geosci. Model Dev. 12, 1443–1475 (2019).
Eyring, V. et al. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 9, 1937–1958 (2016).
Beszczynska-Möller, A., Fahrbach, E., Schauer, U. & Hansen, E. Variability in Atlantic water temperature and transport at the entrance to the Arctic Ocean, 1997–2010. ICES J. Mar. Sci. 69, 852–863 (2012).
Sutton, T. T. Vertical ecology of the pelagic ocean: classical patterns and new perspectives. J. Fish. Biol. 83, 1508–1527 (2013).
Richter, I. Climate model biases in the eastern tropical oceans: causes, impacts and ways forward. WIREs Clim. Change 6, 345–358 (2015).
Pozo Buil, M. et al. A dynamically downscaled ensemble of future projections for the California Current System. Front. Mar. Sci. 8, 612874 (2021).
Leonard, M. et al. A compound event framework for understanding extreme impacts. WIREs Clim. Change 5, 113–128 (2014).
Kwiatkowski, L. et al. Twenty-first century ocean warming, acidification, deoxygenation, and upper-ocean nutrient and primary production decline from CMIP6 model projections. Biogeosciences 17, 3439–3470 (2020).
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245 (2013).
Cheng, L., Abraham, J., Hausfather, Z. & Trenberth, K. E. How fast are the oceans warming? Science 363, 128–129 (2019).
Hawkins, E. & Sutton, R. Time of emergence of climate signals. Geophys. Res. Lett. 39, L01702 (2012).
Stuart-Smith, R. D., Edgar, G. J., Barrett, N. S., Kininmonth, S. J. & Bates, A. E. Thermal biases and vulnerability to warming in the world’s marine fauna. Nature 528, 88–92 (2015).
Filbee-Dexter, K. et al. Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Sci. Rep. 10, 13388 (2020).
Román-Palacios, C. & Wiens, J. J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl Acad. Sci. USA 117, 4211–4217 (2020).
Silvy, Y., Guilyardi, E., Sallée, J.-B. & Durack, P. J. Human-induced changes to the global ocean water masses and their time of emergence. Nat. Clim. Change 10, 1030–1036 (2020).
Cheng, L., Zheng, F. & Zhu, J. Distinctive ocean interior changes during the recent warming slowdown. Sci. Rep. 5, 14346 (2015).
Brito-Morales, I. et al. Climate velocity reveals increasing exposure of deep-ocean biodiversity to future warming. Nat. Clim. Change 10, 576–581 (2020).
Frölicher, T. L. & Laufkötter, C. Emerging risks from marine heat waves. Nat. Commun. 9, 650 (2018).
Oliver, E. C. J. et al. Marine Heatwaves. Ann. Rev. Mar. Sci. 13, 313–342 (2021).
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (2005).
Chaudhary, C., Richardson, A. J., Schoeman, D. S. & Costello, M. J. Global warming is causing a more pronounced dip in marine species richness around the equator. Proc. Natl Acad. Sci. USA 118, e2015094118 (2021).
Burrows, M. T. et al. Ocean community warming responses explained by thermal affinities and temperature gradients. Nat. Clim. Change 9, 959–963 (2019).
IPCC Climate Change 2022: Impacts, Adaptation, and Vulnerability (eds Pörtner, H.-O. et al.) (Cambridge Univ. Press, 2022).
Cahill, A. E. et al. How does climate change cause extinction? Proc. R. Soc. B280, 20121890 (2013).
Hastings, R. A. et al. Climate change drives poleward increases and equatorward declines in marine species. Curr. Biol. 30, 1572–1577.e2 (2020).
Jorda, G. et al. Ocean warming compresses the three-dimensional habitat of marine life. Nat. Ecol. Evol. 4, 109–114 (2020).
Dulvy, N. K. et al. Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J. Appl. Ecol. 45, 1029–1039 (2008).
Thatje, S. Climate warming affects the depth distribution of marine ectotherms. Mar. Ecol. Prog. Ser. 660, 233–240 (2021).
Manuel, S. A., Coates, K. A., Kenworthy, W. J. & Fourqurean, J. W. Tropical species at the northern limit of their range: composition and distribution in Bermuda’s benthic habitats in relation to depth and light availability. Mar. Environ. Res. 89, 63–75 (2013).
Peck, L. S., Webb, K. E. & Bailey, D. M. Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct. Ecol. 18, 625–630 (2004).
Peck, L. S., Morley, S. A., Richard, J. & Clark, M. S. Acclimation and thermal tolerance in Antarctic marine ectotherms. J. Exp. Biol. 217, 16–22 (2014).
Walsh, J. E. Climate of the Arctic marine environment. Ecol. Appl. 18, S3–S22 (2008).
Storch, D., Menzel, L., Frickenhaus, S. & Pörtner, H.-O. Climate sensitivity across marine domains of life: limits to evolutionary adaptation shape species interactions. Glob. Change Biol. 20, 3059–3067 (2014).
Araújo, M. B. et al. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219 (2013).
Pörtner, H. O., Peck, L. & Somero, G. Thermal limits and adaptation in marine Antarctic ectotherms: an integrative view. Philos. Trans. R. Soc. B 362, 2233–2258 (2007).
Qu, Y.-F. & Wiens, J. J. Higher temperatures lower rates of physiological and niche evolution. Proc. R. Soc. B 287, 20200823 (2020).
Cohen, D.M., Inada, T., Iwamoto, T. and Scialabba, N. FAO Species Catalogue, Vol. 10. Gadiform Fishes of the World (Order Gadiformes) (FAO, 1990).
Strand, E. & Huse, G. Vertical migration in adult Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 64, 1747–1760 (2007).
Frölicher, T. L., Fischer, E. M. & Gruber, N. Marine heatwaves under global warming. Nature 560, 360–364 (2018).
Wernberg, T. et al. Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172 (2016).
Smale, D. A. et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change 9, 306–312 (2019).
Cheung, W. W. L. & Frölicher, T. L. Marine heatwaves exacerbate climate change impacts for fisheries in the northeast Pacific. Sci. Rep. 10, 6678 (2020).
Brierley, A. S. & Kingsford, M. J. Impacts of climate change on marine organisms and ecosystems. Curr. Biol. 19, R602–R614 (2009).
Bijma, J., Pörtner, H.-O., Yesson, C. & Rogers, A. D. Climate change and the oceans—what does the future hold? Mar. Pollut. Bull. 74, 495–505 (2013).
Jackson, J. B. C. et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637 (2001).
Duarte, C. M. et al. The soundscape of the Anthropocene ocean. Science 371, eaba4658 (2021).
Rochman, C. M. & Hoellein, T. The global odyssey of plastic pollution. Science 368, 1184–1185 (2020).
Gruber, N., Boyd, P. W., Frölicher, T. L. & Vogt, M. Biogeochemical extremes and compound events in the ocean. Nature 600, 395–407 (2021).
Madec, G. et al. NEMO ocean engine. Zenodo https://www.earth-prints.org/handle/2122/13309 (2017).
Mathiot, P., Jenkins, A., Harris, C. & Madec, G. Explicit representation and parametrised impacts of under ice shelf seas in the z∗- coordinate ocean model NEMO 3.6. Geosci. Model Dev. 10, 2849–2874 (2017).
Dai, A. & Bloecker, C. E. Impacts of internal variability on temperature and precipitation trends in large ensemble simulations by two climate models. Clim. Dyn. 52, 289–306 (2019).
Deser, C., Phillips, A., Bourdette, V. & Teng, H. Uncertainty in climate change projections: the role of internal variability. Clim. Dyn. 38, 527–546 (2012).
Middag, R. et al. Intercomparison of dissolved trace elements at the Bermuda Atlantic Time Series station. Mar. Chem. 177, 476–489 (2015).
Welch, B. L. The generalization of Student’s’ problem when several different population variances are involved. Biometrika 34, 28 (1947).
Lenoir, J. et al. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 4, 1044–1059 (2020).
Janzen, D. H. Why mountain passes are higher in the Tropics. Am. Nat. 101, 233–249 (1967).
Seebacher, F., White, C. R. & Franklin, C. E. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66 (2015).
Hoffmann, A. A. & Sgrò, C. M. Climate change and evolutionary adaptation. Nature 470, 479–485 (2011).
Sandblom, E. et al. Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat. Commun. 7, 11447 (2016).
Tewksbury, J. J., Huey, R. B. & Deutsch, C. A. Putting the heat on tropical animals. Science 320, 1296–1297 (2008).
Dahlke, F. T., Wohlrab, S., Butzin, M. & Pörtner, H.-O. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369, 65–70 (2020).
Acknowledgements
This work was supported by the European Union’s Horizon 2020 research and innovation programme with the TRIATLAS project under the grant agreement number 817578 (Y.S.-F. and R.S.), the COMFORT project under the grant agreement number 820989 (Y.S.-F. and R.S.) and the ESM2025 project under the grant agreement number 101003536 (R.S.). The work reflects only the authors’ view; the European Commission and their executive agency are not responsible for any use that may be made of the information the work contains. We thank L. Kwiatkowski, S. Berthet and E. Sánchez for comments on pre-submission drafts of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.S.-F. and R.S. conceived the study, developed the datasets, performed the computations and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Climate Change thanks the anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Observed and simulated daily temperature across the six stations.
Depth-time plots of daily ocean temperature anomalies from the surface to 1000 m. Anomalies are computed for the full observational records by removing the daily climatological temperature to daily temperature. They are indicated for both observations and CNRM-ESM2-1. The observational mask in space and time is applied to model data. Red/blue colours indicate warmer/cooler daily temperature variations with respect to the daily climatological temperature. Profiles of the full observational period are given for both observations (orange) and the model ensemble mean (black). Fifteen model ensemble members are also included (grey). Blank space indicates lack of observational data.
Extended Data Fig. 2 Thermal range developments in response to climate change.
Schematics of possible developments of thermal ranges as a consequence of concomitant changes in their lower (Tmin) and upper (Tmax) bounds in response to climate change. Both changes that reduce or increase the upper or lower limit, or changes in both, will perturb the thermal range. These changes can either expand, contract, or shift it toward cooling or warming, possibly leading to a redistribution or collapse of the original marine habitat. Colour code refers to those shown in Fig. 4.
Extended Data Fig. 3 Profiles of thermal range boundaries and detected trends at each station.
Profiles of lower and upper boundaries of the thermal range are presented with anomalies of Tmin (bluish colours) and Tmax (reddish colours) relative to temperature mean over the period of available observations as simulated by CNRM-ESM2-1, respectively. Dashed lines demarcate the upper epipelagic, lower epipelagic and mesopelagic layers. Within these layers, numbers indicate mean trends per decade as derived from observations (grey) and as simulated by the model (black). Only significant trends with respect to internal climate variability are shown. Positive (negative) values indicate warming (cooling) trends.
Extended Data Fig. 4 Probability density function (pdf) of trends for Tmin (a) and Tmax (b) over the observational period for each station.
Trends are estimated from a 100 randomly selected observational period-long time series of the piControl simulation. The pdf over upper epipelagic, lower epipelagic and mesopelagic layers are displayed in blue. Trends derived from observations (orange) and simulated by the model (black) during the observational period are given with vertical lines. Empirical p-value as derived from the comparison of the observed and modelled trends against the distribution of the piControl trends are presented in Supplementary Table 3.
Extended Data Fig. 5 Examples of Tmin- and Tmax-based ToE computations at the station HOT-01.
(a) Examples of the computation of the timing for which future Tmin will be warmer than the current Tmidpoint. At each depth level, ToE is considered as the year at which a spline for the simulation (1990 to 2100) time series of Tmin surpasses the mean of a spline ± climate variability for the current period (1990 to 2020) Tmidpoint. SSP5-8.5 is considered in this example. Climate variability is considered as the 5th and 95th percentiles of a 100 randomly selected 30 years period time series of Tmidpoint as simulated by fifteen samples of the piControl simulation. (b) Examples of the computation of the timing for which future Tmin will be warmer than the current Tmax. At each depth level, ToE is considered as the year at which a spline for the simulation (1990 to 2100) time series of Tmin surpasses the mean of a spline ± climate variability for the current period (1990 to 2020) Tmax. SSP5-8.5 is considered in this example. Climate variability is considered as the 5th and 95th percentiles of a 100 randomly selected 30 years period time series of Tmax as simulated by fifteen samples of the piControl simulation. (c) Examples of the computation of the timing for which future Tmax emerges from current natural variability. At each depth level, ToE is considered as the year at which a spline for the simulation (1990 to 2100) time series of Tmax surpasses the mean of a spline + climate variability for the current period (1990 to 2020) Tmax. SSP5-8.5 is considered in this example. Climate variability is considered as twice the standard deviation of a 100 randomly selected 30 years period time series of Tmax as simulated by fifteen samples of the piControl simulation.
Extended Data Fig. 6 End-of-the-century thermal ranges resulting from concomitant changes in their lower and upper bounds in response to climate change considering SSP5-8.5.
Profiles illustrate temperature anomalies for Tmin and Tmax with respect to the mean over last years of the historical simulation (1990 to 2014) for the historical (1990 to 2014) and end-of-the-century (2080 to 2100) periods. Reddish (bluish) shading areas indicate ocean layers where end-of-the-century Tmin and Tmax are warmer (cooler) than the historical counterparts. Numbers indicate their anomalies (in °C). Climate Novelty profiles are given in the right-hand sided boxes. Dashed lines demarcate the upper epipelagic, lower epipelagic and mesopelagic layers.
Extended Data Fig. 7 End-of-the-century number of days and intensity of marine heatwaves (MHWs) anomalies with respect to historical period.
MHWs anomalies are computed as the difference between the end-of-the-century (2080 to 2100) and the historical period (1990 to 2014) number of days (a) and maximum intensity (b). Differences are given for upper epipelagic, lower epipelagic and mesopelagic waters. High (SSP5-8.5), moderate (SSP2-4.5), and low (SSP1-2.6) emission scenarios are displayed.
Extended Data Fig. 8 End-of-the-century thermal ranges resulting from concomitant changes in their lower and upper bounds in response to climate change considering SSP2-4.5.
Profiles illustrate temperature anomalies for Tmin and Tmax with respect to the mean over last years of the historical simulation (1990 to 2014) for the historical (1990 to 2014) and end-of-the-century (2080 to 2100) periods. Reddish (bluish) shading areas indicate ocean layers where end-of-the-century Tmin and Tmax are warmer (cooler) than the historical counterparts. Numbers indicate their anomalies (in °C). Climate Novelty profiles are given in the right-hand sided boxes. Dashed lines demarcate the upper epipelagic, lower epipelagic and mesopelagic layers.
Extended Data Fig. 9 End-of-the-century thermal ranges resulting from concomitant changes in their lower and upper bounds in response to climate change considering SSP1-2.6.
Profiles illustrate temperature anomalies for Tmin and Tmax with respect to the mean over last years of the historical simulation (1990 to 2014) for the historical (1990 to 2014) and end-of-the-century (2080 to 2100) periods. Reddish (bluish) shading areas indicate ocean layers where end-of-the-century Tmin and Tmax are warmer (cooler) than the historical counterparts. Numbers indicate their anomalies (in °C). Climate Novelty profiles are given in the right-hand sided boxes. Dashed lines demarcate the upper epipelagic, lower epipelagic and mesopelagic layers.
Extended Data Fig. 10 Maps of the changes in thermal ranges at the end of the century resulting from concomitant changes in both lower and upper boundaries.
These maps provide a geographical representation of the right hand sided boxes as shown in Fig. 4 for the high (SSP5-8.5), moderate (SSP2-4.5), and low (SSP1-2.6) emission scenarios. Changes in thermal ranges are averaged for the upper epipelagic, lower epipelagic, and mesopelagic layers, consistently with Fig. 4.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–7 and Tables 1–3.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santana-Falcón, Y., Séférian, R. Climate change impacts the vertical structure of marine ecosystem thermal ranges. Nat. Clim. Chang. 12, 935–942 (2022). https://doi.org/10.1038/s41558-022-01476-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-022-01476-5
This article is cited by
-
Early detection of anthropogenic climate change signals in the ocean interior
Scientific Reports (2023)
-
Marine biodiversity exposed to prolonged and intense subsurface heatwaves
Nature Climate Change (2023)