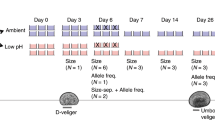
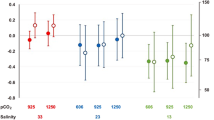
Abstract
Ocean acidification may have deleterious effects on many species, but anticipating long-term changes in the abundance of populations will require an understanding of ocean acidification as an evolutionary force. Here, I show that ocean acidification alters natural selection on offspring size and is likely to drive contemporary evolution. In a detailed study of a coastal fish species (California grunion), I demonstrate that larval mortality is highly sensitive to ocean acidification and that mortality rates are lower for larger larvae. However, these effects are countered by tradeoffs between offspring size and number, suggesting that measurements of maternal fitness are critical for quantifying selection through ocean acidification. Measurements of selection and genetic variation were used to project the evolution of larval size as seawater conditions changed incrementally over many decades. Results for California grunion suggest that contemporary evolution may offset the projected decline in reproductive success by about 50%.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data from this study are available through the Dryad digital repository (https://doi.org/10.5061/dryad.0gb5mkm3w)55.
Code availability
R scripts used to analyze variation and natural selection on larval size and to project evolutionary responses of larvae to ocean acidification are available at Zenodo and accessible from the Dryad link55.
References
Sunday, J. M., Crim, R. N., Harley, C. D. G. & Hart, M. W. Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6, e22881 (2011).
Kelly, M. W. & Hofmann, G. E. Adaptation and the physiology of ocean acidification. Funct. Ecol. 27, 980–990 (2013).
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M. & Marshall, D. J. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500 (2013).
Reusch, T. B. H. Climate change in the oceans: evolutionary versus phenotypically plastic responses of marine animals and plants. Evol. Appl. 7, 104–122 (2014).
Sunday, J. M. et al. Evolution in an acidifying ocean. Trends Ecol. Evol. 29, 117–125 (2014).
Kroeker, K. J., Kordas, R. L., Crim, R. N. & Singh, G. G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434 (2010).
Przeslawski, R., Byrne, M. & Mellin, C. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Change Biol. 21, 2122–2140 (2015).
Cattano, C., Claudet, J., Domenici, P. & Milazzo, M. Living in a high CO2 world: a global meta-analysis shows multiple trait-mediated fish responses to ocean acidification. Ecol. Monogr. 88, 320–335 (2018).
Lohbeck, K., Riebesell, U. & Reusch, T. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5, 346–351 (2012).
Dam, H. G. et al. Rapid, but limited, zooplankton adaptation to simultaneous warming and acidification. Nat. Clim. Change 11, 780–786 (2021).
Kelly, M. W., Padilla-Gamiño, J. L. & Hofmann, G. E. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob. Change Biol. 19, 2536–2546 (2013).
Pespeni, M. H. et al. Evolutionary change during experimental ocean acidification. Proc. Natl Acad. Sci. USA 110, 6937–6942 (2013).
Foo, S. A., Dworjanyn, S. A., Poore, A. G. B., Harianto, J. & Byrne, M. Adaptive capacity of the sea urchin Heliocidaris erythrogramma to ocean change stressors: responses from gamete performance to the juvenile. Mar. Ecol. Prog. Ser. 556, 161–172 (2016).
Malvezzi, A. J. et al. A quantitative genetic approach to assess the evolutionary potential of a coastal marine fish to ocean acidification. Evol. Appl. 8, 352–362 (2015).
Bitter, M. C., Kapsenberg, L., Gattuso, J.-P. & Pfister, C. A. Standing genetic variation fuels rapid adaptation to ocean acidification. Nat. Commun. 10, 5821 (2019).
Falconer, D. S. & Mackay, T. F. C. Introduction to Quantitative Genetics 4th edn (Pearson Prentice Hall, 1996).
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Oxford Univ. Press, 1998).
Ishimatsu, A., Hayashi, M. & Kikkawa, T. Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–302 (2008).
Melzner, F. et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331 (2009).
Timothy A. Mousseau and Charles W. Fox. Maternal Effects as Adaptations 178–201 (Oxford Univ. Press, 1998).
Marshall, D., Allen, R. & Crean, A. The ecological and evolutionary importance of maternal effects in the sea. Oceanogr. Mar. Biol. 46, 203–250 (2008).
Tasoff, A. J. & Johnson, D. W. Can larvae of a marine fish adapt to ocean acidification? Evaluating the evolutionary potential of California grunion (Leuresthes tenuis). Evol. Appl. 12, 560–571 (2019).
Smith, C. C. & Fretwell, S. D. The optimal balance between size and number of offspring. Am. Nat. 108, 499–506 (1974).
Shimada, Y., Shikano, T., Murakami, N., Tsuzaki, T. & Seikai, T. Maternal and genetic effects on individual variation during early development in Japanese flounder Paralichthys olivaceus. Fish. Sci. 73, 244–249 (2007).
Johnson, D. W., Christie, M. R. & Moye, J. Quantifying evolutionary potential of marine fish larvae: heritability, selection, and evolutionary constraints. Evolution 64, 2614–2628 (2010).
Miles, C. M., Hadfield, M. G. & Wayne, M. L. Heritability for egg size in the serpulid polychaete Hydroides elegans. Mar. Ecol. Prog. Ser. 340, 155–162 (2007).
Iguchi, K. & Yamaguchi, M. Adaptive significance of inter- and intrapopulational egg size variation in ayu Plecoglossus altivelis (osmeridae). Copeia 1994, 184–190 (1994).
Marshall, D. J. & Keough, M. J. Effects of settler size and density on early post-settlement survival of Ciona intestinalis in the field. Mar. Ecol. Prog. Ser. 259, 139–144 (2003).
González-Ortegón, E. & Giménez, L. Environmentally mediated phenotypic links and performance in larvae of a marine invertebrate. Mar. Ecol. Prog. Ser. 502, 185–195 (2014).
Pan, T.-C. F., Applebaum, S. L. & Manahan, D. T. Experimental ocean acidification alters the allocation of metabolic energy. Proc. Natl Acad. Sci. USA 112, 4696–4701 (2015).
Rollinson, N. & Hutchings, J. A. Environmental quality predicts optimal egg size in the wild. Am. Nat. 181, 76–90 (2013).
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Oxford Univ. Press, 1998).
Munday, P. L. Transgenerational acclimation of fishes to climate change and ocean acidification. F1000Prime Rep. 6, 99 (2014).
Murray, C. S., Malvezzi, A., Gobler, C. J. & Baumann, H. Offspring sensitivity to ocean acidification changes seasonally in a coastal marine fish. Mar. Ecol. Prog. Ser. 504, 1–11 (2014).
Baumann, H. Experimental assessments of marine species sensitivities to ocean acidification and co-stressors: how far have we come? Can. J. Zool. 97, 399–408 (2019).
Chevin, L.-M., Lande, R. & Mace, G. M. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 (2010).
Bell, G. Evolutionary rescue and the limits of adaptation. Phil. Trans. R. Soc. B 368, p20120080 (2013).
Carlson, S. M., Cunningham, C. J. & Westley, P. A. H. Evolutionary rescue in a changing world. Trends Ecol. Evol. 29, 521–530 (2014).
Smyder, E. A., Martin, K. L. M. & Gatten, R. E. Jr Temperature effects on egg survival and hatching during the extended incubation period of California grunion, Leuresthes tenuis. Copeia 2002, 313–320 (2002).
Barneche, D. R., Robertson, D. R., White, C. R. & Marshall, D. J. Fish reproductive-energy output increases disproportionately with body size. Science 360, 642–645 (2018).
Van Noordwijk, A. J. & de Jong, G. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142 (1986).
Davidson, C. Spatial and Temporal Variability of Coastal Carbonate Chemistry in the Southern California Region. MSc thesis, Univ. California, San Diego (2015).
Jones, J. M., Sweet, J., Brzezinski, M. A., McNair, H. M. & Passow, U. Evaluating carbonate system algorithms in a nearshore system: does total alkalinity matter? PLoS ONE 11, e0165191 (2016).
Gruber, N. et al. Rapid progression of ocean acidification in the California current system. Science 337, 220–223 (2012).
Turi, G., Lachkar, Z., Gruber, N. & Münnich, M. Climatic modulation of recent trends in ocean acidification in the California current system. Environ. Res. Lett. 11, 014007 (2016).
Northcott, D. et al. Impacts of urban carbon dioxide emissions on sea-air flux and ocean acidification in nearshore waters. PLoS ONE 14, e0214403 (2019).
Rausher, M. D. The measurement of selection on quantitative traits: biases due to environmental covariances between traits and fitness. Evolution 46, 616–626 (1992).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S (Springer, 2002).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
Kruuk, L. E. B. Estimating genetic parameters in natural populations using the animal model. Phil. Trans. R. Soc. B 359, 873–890 (2004).
Wilson, A. J. et al. An ecologist’s guide to the animal model. J. Anim. Ecol. 79, 13–26 (2010).
Hadfield, J. D. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, (2010).
Heidelberger, P. & Welch, P. D. Simulation run length control in the presence of an initial transient. Oper. Res. 31, 1109–1144 (1983).
Clark, F. N. The Life History of Leuresthes Tenuis, an Atherine Fish with Tide Controlled Spawning Habits Fish Bulletin No. 10 (California Department of Fish and Game, 1925).
Johnson, D.W. Data from: Selection on offspring size and contemporary evolution under ocean acidification. Dryad https://doi.org/10.5061/dryad.0gb5mkm3w (2022)
Acknowledgements
Special thanks to A. Tasoff, E. Siegfried, J. Chhor, C. Shelley, J. Paz, B. McCann, T. Morales, S. Parrott and C. Powell for their assistance with data collection. I also thank Y. Ralph and the staff of the CSULB Marine Lab for logistical support. This study was funded by NSF award OCE-1948975 to D.W.J.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Climate Change thanks Miguel Baltazar-Soares, Andrea Frommel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Change in larval size throughout reproductive season.
Variation in larval size by month and year. Solid horizontal lines indicate the medians. Boxes represent the interquartile range and vertical lines extend to the largest and smallest values within 1.5 times the interquartile range.
Extended Data Fig. 2 Projected evolution of larval size and relative fitness using the Breeder’s equation.
Projected evolution of larval size and effects on relative reproductive success using a two-sex version of the Breeder’s equation. Solid line describes the projected reproductive success in the absence of evolution and the dot-and-dashed line represents reproductive success when larval size is allowed to evolve according to the patterns of selection and heritability described in this study. Thin dashed lines and shaded area represent the 95% Confidence Intervals for the projections with and without evolutionary responses.
Supplementary information
Supplementary Information
Supplementary Text 1 and 2, Supplementary Tables 1–3 and Supplementary Figs. 1–4.
Rights and permissions
About this article
Cite this article
Johnson, D.W. Selection on offspring size and contemporary evolution under ocean acidification. Nat. Clim. Chang. 12, 757–760 (2022). https://doi.org/10.1038/s41558-022-01425-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-022-01425-2