Abstract
Attempts to link physiological thermal tolerance to global species distributions have relied on lethal temperature limits, yet many organisms lose fertility at sublethal temperatures. Here we show that, across 43 Drosophila species, global distributions better match male-sterilizing temperatures than lethal temperatures. This suggests that species distributions may be determined by thermal limits to reproduction, not survival, meaning we may be underestimating the impacts of climate change for many organisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All novel data underlying the analyses and figures presented in this paper are available from Dryad at https://doi.org/10.5061/dryad.f4qrfj6tt.
Code availability
Analysis R codes are available upon request from the corresponding authors.
References
Pecl, G. T. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214 (2017).
Kearney, M. & Porter, W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 12, 334–350 (2009).
Nowakowski, A. J. et al. Thermal biology mediates responses of amphibians and reptiles to habitat modification. Ecol. Lett. 21, 345–355 (2018).
Metelmann, S. et al. The UK’s suitability for Aedes albopictus in current and future climates. J. R. Soc. Interface 16, 20180761 (2019).
Kellermann, V. et al. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl Acad. Sci. USA 109, 16228–16233 (2012).
Lancaster, L. T. & Humphreys, A. M. Global variation in the thermal tolerances of plants. Proc. Natl Acad. Sci. USA 117, 13580–13587 (2020).
Sunday, J. M. et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615 (2014).
Rezende, E. L., Bozinovic, F., Szilàgyi, A. & Santos, M. Predicting temperature mortality and selection in natural Drosophila populations. Science 369, 1242–1245 (2020).
Jørgensen, L. B., Malte, H. & Overgaard, J. How to assess Drosophila heat tolerance: unifying static and dynamic tolerance assays to predict heat distribution limits. Funct. Ecol. 33, 629–642 (2019).
Rezende, E. L., Castañeda, L. E. & Santos, M. Tolerance landscapes in thermal ecology. Funct. Ecol. 28, 799–809 (2014).
Terblanche, J. S. & Hoffmann, A. A. Validating measurements of acclimation for climate change adaptation. Curr. Opin. Insect Sci. 41, 7–16 (2020).
Walsh, B. S. et al. The impact of climate change on fertility. Trends Ecol. Evol. 34, 249–259 (2019).
Sage, T. L. et al. The effect of high temperature stress on male and female reproduction in plants. Field Crops Res. 182, 30–42 (2015).
Sales, K. et al. Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 9, 4771 (2018).
Porcelli, D., Gaston, K. J., Butlin, R. K. & Snook, R. R. Local adaptation of reproductive performance during thermal stress. J. Evol. Biol. 30, 422–429 (2016).
Saxon, A. D., O’Brien, E. K. & Bridle, J. R. Temperature fluctuations during development reduce male fitness and may limit adaptive potential in tropical rainforest Drosophila. J. Evol. Biol. 31, 405–415 (2018).
Breckels, R. D. & Neff, B. D. The effects of elevated temperature on the sexual traits, immunology and survivorship of a tropical ectotherm. J. Exp. Biol. 216, 2658–2664 (2013).
Paxton, C. W., Baria, M. V. B., Weis, V. M. & Harii, S. Effect of elevated temperature on fecundity and reproductive timing in the coral Acropora digitifera. Zygote 24, 511–516 (2016).
Hurley, L. L., McDiarmid, C. S., Friesen, C. R., Griffith, S. C. & Rowe, M. Experimental heatwaves negatively impact sperm quality in the zebra finch. Proc. R. Soc. Lond. B 285, 20172547 (2018).
Yogev, L. et al. Seasonal variations in pre‐ and post‐thaw donor sperm quality. Hum. Reprod. 19, 880–885 (2004).
Terblanche, J. S., Deere, J. A., Clusella Trullas, S., Janion, C. & Chown, S. L. Critical thermal limits depend on methodological context. Proc. R. Soc. Lond. B 274, 2935–2942 (2007).
Ives, A. R. R2s for correlated data: phylogenetic models, LMMs, and GLMMs. Syst. Biol. 68, 234–251 (2019).
Dillon, M. E., Wang, G., Garrity, P. A. & Huey, R. B. Thermal preference in Drosophila. J. Therm. Biol. 34, 109–119 (2009).
Tratter-Kinzner, M. et al. Is temperature preference in the laboratory ecologically relevant for the field? The case of Drosophila nigrosparsa. Glob. Ecol. Conserv. 18, e00638 (2019).
van Heerwaarden, B. & Sgrò, C. M. Male fertility thermal limits predict vulnerability to climate warming. Nat. Commun. 12, 2214 (2021).
Acknowledgements
We acknowledge N. Mannion and A. Sims for assistance with experiments, B. Longdon, K. Roberts and M. Garlovsky for supplying us with flies and R. Connell for designing three-dimensionally printed equipment. We thank P. Rohner and S. Lüpold for sharing their phylogenetic tree file. Funding was provided by Nature and Environment Research Council grant NE/P002692/1 and European Society for Evolutionary Biology Special Topic Network “The evolutionary ecology of thermal fertility limits” to T.A.R.P., A.J.B., A.A.H. and R.R.S, Swiss National Science Foundation P300PA_177830 to A.M. and the National Institute for Health Research: Health Protection Research Unit into Emerging Zoonotic Infections to S.M.
Author information
Authors and Affiliations
Contributions
The study was conceived by T.A.R.P., A.J.B., R.R.S., A.A.H. and S.R.P. The experimental methodology and data collection were developed and conducted by S.R.P., B.S.W. and N.W. Data from existing open sources were curated by S.R.P. and S.M. Statistical analysis and visualization was conducted by S.R.P., A.M. and S.M. The manuscript was drafted by S.R.P., T.A.R.P., A.J.B., R.R.S. and A.A.H. All authors reviewed and commented on the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Enrico Rezende, Ramakrishnan Vasudeva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
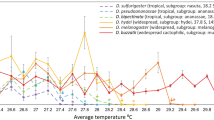
Extended Data Fig. 1 TFL80 and LT80 of Drosophila immediately after heat stress.
Several species of Drosophila lose 80% fertility at cooler-than-lethal temperatures immediately following heat-shock. LT80 (black circles) and TFL80 (pink circles). Thick, pale pink bars connect values collected form the same species to aid visualization. Errors for both measures are 95% confidence intervals generated from dose response model estimates. Fertility loss measured as ability to sire any offspring between 1–6 days post heat-stress.
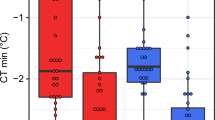
Extended Data Fig. 2 Thermal safety margins for 43 Drosophila species.
a, Central safety margins calculated using the physiological limit minus the mean maximum temperature across all recorded locations for each species. b, Distribution safety margins based on the physiological limit minus mean maximum air temperature + 1SD across all recorded locations for each species to capture populations at the boundary of the thermal range5. Grey squares and line are safety margins calculated using CTMAX values, black circles and lines are based on LT80, and pink points and lines are based on TFL80 measured 7-days after heat stress. TFL80 measured at 7 days post-heat stress predicts mean central safety margins of 4.97 ± 2.42 °C. Distribution safety margins are reduced from a mean of 2.38 ± 3.46 °C when based on LT80, to 1.23 ± 3.04 °C when based on TFL80. Several species have populations that currently experience temperatures that are hotter than their fertility limits. It would be interesting to know whether species that have small or negative safety margins avoid breeding at the hottest time of year, perhaps by aestivating as adults or surviving as eggs or pupa. Latitude shown here is the mean absolute latitude that is the sign of negative latitudes has been removed to give relative distance from the equator. Fitted lines are predictions from models in which the limit type (TFL, LT or CTMAX) and latitude2 are fixed effects and species identity is a random effect to account for repeated measures across species (Supplementary Table 4).
Extended Data Fig. 3 Repeatability of LT80.
Repeatability was high across the 22 Drosophila species tested. Left panel: There was no significant difference in the estimate of LT80 between full runs and control repeats in any of these species (significance measured as non-overlapping CIs of point estimates of LT80). Pink points = estimate from single species assay, gold point = estimate from simultaneous multispecies control assay. Right panel: The correlation between the two independent measurements of LT80 across these species was strongly positive and explained a high degree of variation in the data (coefficient = 1.06, R2 = 0.96).
Extended Data Fig. 4 Repeatability of TFL80 for Drosophila virilis.
We independently repeated the TFL80 assay for one species in which we found a significant difference between LT80 and TFL80. Two independent runs of sexually mature males were conducted by two researchers 6 months apart. Red = original data used to calculate TFL80 for D. virilis in this manuscript, blue = repeated assay. Dashed lines intersect at 80% threshold. Line fits predicted by 3-parameter dose-response model. The fly stock and equipment were identical.
Extended Data Fig. 5 Repeatability of knockdown CTMAX.
Left panel; mean CTMAX temperature recorded from species’ independent assays (gold points), and in mixed species control blocks (pink points). There was no global significant difference between CTMAX estimates across all species (Block: F1,831 = 3.246, P = 0.072). However, a significant experimental block*species interaction was found for 4 of the 41 species tested (interaction term: F40,831 = 3.7589, P <0,001); Drosophila sechelia, D. nasuta, D. yakuba & D. tiesseri all scored significantly higher CTMAX in our mixed species control block than in their own individual CTMAX assays. Right panel: The correlation between estimated CTMAX values from both blocks was strongly positive (coefficient = 1.07, F1,37 = 309.84, P <0.001), and explained 89% of the variation.
Supplementary information
Supplementary Information
Supplementary Methods and Tables 1–5.
Rights and permissions
About this article
Cite this article
Parratt, S.R., Walsh, B.S., Metelmann, S. et al. Temperatures that sterilize males better match global species distributions than lethal temperatures. Nat. Clim. Chang. 11, 481–484 (2021). https://doi.org/10.1038/s41558-021-01047-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-021-01047-0
This article is cited by
-
Detrimental impact of a heatwave on male reproductive behaviour and fertility
acta ethologica (2024)
-
Heat-induced hormesis in longevity is linked to heat-stress sensitivity across laboratory populations from diverse altitude of origin in Drosophila buzzatii
Biogerontology (2024)
-
It is hot and cold here: the role of thermotolerance in the ability of spiders to colonize tree plantations in the southern Atlantic Forest
Oecologia (2024)
-
Slow and population specific evolutionary response to a warming environment
Scientific Reports (2023)
-
Chronic exposure to warm temperature causes low sperm abundance and quality in Drosophila melanogaster
Scientific Reports (2023)