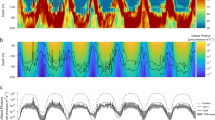
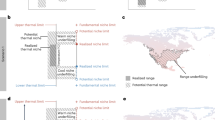
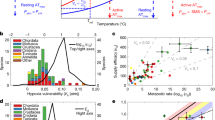
Abstract
Seasonality in light becomes increasingly extreme at high latitudes, both in terms of the diel light–dark cycle and the duration of light summers and dark winters. In contrast to temperature, this latitudinal gradient in light seasonality is not affected by climate change. A key question is therefore whether light may act as a fixed constraint on warming-driven redistributions of organisms at high latitudes. One answer is provided by studying mechanistic models of visual foraging and temperature-driven physiology along latitudinal gradients to project where populations survive and acquire resources to reproduce, and where they demise. Here we contrast such models for two widespread planktivorous fish types. We identify two processes through which seasonality in light can act as a barrier to poleward range expansions at high latitudes: (1) longer dark winters lead to greater depletion of overwinter energy stores and (2) a longer duration of midnight sun entails higher foraging-related predation mortality.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The GIN Seas Regional Climatology with temperature and salinity is freely available online53. The zooplankton prey field was based on published literature values (see Supplementary Section M4 Zooplankton for references), generated in the mesopelagic fish model (code is available, see below), and used as input in the epipelagic fish model. The data used as input in the mesopelagic fish model are available in Supplementary Table 4 and the input data for the epipelagic fish model are available in Supplementary Tables 2 and 3.
References
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925 (2013).
Chen, I. C., Hill, J. K., Ohlemüller, R., Roy, D. B. & Thomas, C. D. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (2011).
Lenoir, J. & Svenning, J.-C. Climate-related range shifts—a global multidimensional synthesis and new research directions. Ecography 38, 15–28 (2015).
Robinson, L. M. et al. Pushing the limits in marine species distribution modelling: lessons from the land present challenges and opportunities. Glob. Ecol. Biogeogr. 20, 789–802 (2011).
Guisan, A. et al. Making better biogeographical predictions of species’ distributions. J. Appl. Ecol. 43, 386–392 (2006).
Wilson, R. P., Culik, B., Coria, N. R., Adelung, D. & Spairani, H. J. Foraging rhythms in Adélie penguins (Pygoscelis adeliae) at Hope Bay, Antarctica; determination and control. Polar Biol. 10, 161–165 (1989).
Aksnes, D. & Utne, A. C. W. A revised model of visual range in fish. Sarsia 4827, 37–41 (1997).
Johansen, R., Barrett, R. T. & Pedersen, T. Foraging strategies of great cormorants Phalacrocorax carbo carbo wintering north of the Arctic Circle. Bird Study 48, 59–67 (2001).
Varpe, Ø. Life history adaptations to seasonality. Integr. Comp. Biol. 57, 943–960 (2017).
Poloczanska, E. S. et al. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3, 62 (2016).
Saikkonen, K. et al. Climate change-driven species’ range shifts filtered by photoperiodism. Nat. Clim. Change 2, 239–242 (2012).
Bradshaw, W. E. & Holzapfel, C. M. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 72, 147–166 (2010).
Huffeldt, N. P. Photic barriers to poleward range-shifts. Trends Ecol. Evol. 35, 652–655 (2020).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690 (2012).
Lenoir, J. et al. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 4, 1044–1059 (2020).
Kaartvedt, S. Photoperiod may constrain the effect of global warming in arctic marine systems. J. Plankton Res. 30, 1203–1206 (2008).
Sundby, S., Drinkwater, K. F. & Kjesbu, O. S. The North Atlantic spring-bloom system—where the changing climate meets the winter dark. Front. Mar. Sci. 3, 28 (2016).
Langbehn, T. J. & Varpe, Ø. Sea-ice loss boosts visual search: fish foraging and changing pelagic interactions in polar oceans. Glob. Chang. Biol. 23, 5318–5330 (2017).
Irigoien, X., Klevjer, T. A. & Røstad, A. Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 5, 3271 (2014).
Geoffroy, M. et al. Mesopelagic sound scattering layers of the high Arctic: seasonal variations in biomass, species assemblage, and trophic relationships. Front. Mar. Sci. 6, 364 (2019).
Jobling, M. Fish Bioenergetics (Chapman & Hall, 1994).
Ljungström, G., Claireaux, M., Fiksen, Ø. & Jørgensen, C. Body size adaptions under climate change: zooplankton community more important than temperature or food abundance in model of a zooplanktivorous fish. Mar. Ecol. Prog. Ser. 636, 1–18 (2020).
Enberg, K. et al. Fishing-induced evolution of growth: concepts, mechanisms and the empirical evidence. Mar. Ecol. 33, 1–25 (2012).
Langbehn, T., Aksnes, D., Kaartvedt, S., Fiksen, Ø. & Jørgensen, C. Light comfort zone in a mesopelagic fish emerges from adaptive behaviour along a latitudinal gradient. Mar. Ecol. Prog. Ser. 623, 161–174 (2019).
Røstad, A., Kaartvedt, S. & Aksnes, D. L. Erratum to ‘Light comfort zones of mesopelagic acoustic scattering layers in two contrasting optical environments’ [Deep Sea Res. I 113 (2016) 1–6]. Deep Sea Res. I Oceanogr. Res. Pap. 114, 162–164 (2016).
Røstad, A., Kaartvedt, S. & Aksnes, D. L. Light comfort zones of mesopelagic acoustic scattering layers in two contrasting optical environments. Deep Sea Res. I Oceanogr. Res. Pap. 113, 1–6 (2016).
Clark, C. W. & Levy, D. A. Diel vertical migrations by juvenile sockeye salmon and the antipredation window. Am. Nat. 131, 271–290 (1988).
Scheuerell, M. D. & Schindler, D. E. Diel vertical migration by juvenile sockeye salmon: empirical evidence for the antipredation window. Ecology 84, 1713–1720 (2003).
Boyce, M. S. Seasonality and patterns of natural selection for life histories. Am. Nat. 114, 569–583 (1979).
Roff, D. A. The Evolution of Life Histories: Theory and Analysis (Chapman & Hall, 1992).
Stearns, S. C. The Evolution of Life Histories (Oxford University Press, 1992).
Varpe, Ø., Jørgensen, C., Tarling, G. A. & Fiksen, Ø. The adaptive value of energy storage and capital breeding in seasonal environments. Oikos 118, 363–370 (2009).
Hagen, W. & Auel, H. Seasonal adaptations and the role of lipids in oceanic zooplankton. Zoology 104, 313–326 (2001).
Robinson, N. M., Nelson, W. A., Costello, M. J., Sutherland, J. E. & Lundquist, C. J. A systematic review of marine-based species distribution models (SDMs) with recommendations for best practice. Front. Mar. Sci. 4, 421 (2017).
Jones, M. C. & Cheung, W. W. L. Multi-model ensemble projections of climate change effects on global marine biodiversity. ICES J. Mar. Sci. 72, 741–752 (2015).
García Molinos, J. et al. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change 6, 83–88 (2016).
Cheung, W. W. L. et al. Projecting global marine biodiversity impacts under climate change scenarios. Fish. Fish. 10, 235–251 (2009).
Sinclair, S. J., White, M. D. & Newell, G. R. How useful are species distribution models for managing biodiversity under future climates? Ecol. Soc. 15, 8 (2010).
Twiname, S. et al. A cross-scale framework to support a mechanistic understanding and modelling of marine climate-driven species redistribution, from individuals to communities. Ecography 43, 1764–1778 (2020).
Dullinger, S. et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Change 2, 619–622 (2012).
Bush, A. et al. Incorporating evolutionary adaptation in species distribution modelling reduces projected vulnerability to climate change. Ecol. Lett. 19, 1468–1478 (2016).
Cheung, W. W. L., Dunne, J., Sarmiento, J. L. & Pauly, D. Integrating ecophysiology and plankton dynamics into projected maximum fisheries catch potential under climate change in the Northeast Atlantic. ICES J. Mar. Sci. 68, 1008–1018 (2011).
Saunders, R. A., Collins, M. A., Stowasser, G. & Tarling, G. A. Southern Ocean mesopelagic fish communities in the Scotia Sea are sustained by mass immigration. Mar. Ecol. Prog. Ser. 569, 173–185 (2017).
Audzijonyte, A. et al. Atlantis: a spatially explicit end-to-end marine ecosystem model with dynamically integrated physics, ecology and socio-economic modules. Methods Ecol. Evol. 10, 1814–1819 (2019).
Alerstam, T., Hedenström, A. & Åkesson, S. Long-distance migration: evolution and determinants. Oikos 103, 247–260 (2003).
Nøttestad, L., Giske, J., Holst, J. C. & Huse, G. A length-based hypothesis for feeding migrations in pelagic fish. Can. J. Fish. Aquat. Sci. 56, 26–34 (1999).
Roff, D. A. The evolution of migration and some life history parameters in marine fishes. Environ. Biol. Fishes 22, 133–146 (1988).
Alder, J., Campbell, B., Karpouzi, V., Kaschner, K. & Pauly, D. Forage fish: from ecosystems to markets. Annu. Rev. Environ. Resour. 33, 153–166 (2008).
Houston, A. I. & McNamara, J. M. Models of Adaptive Behaviour: An Approach Based on State (Cambridge University Press, 1999).
Clark, C. W. & Mangel, M. Dynamic State Variable Models in Ecology (Oxford University Press, 2000).
Hoegh-Guldberg, O. et al. in Climate Change 2014: Impacts, Adaptation, and Vulnerability (eds Barros, V. R. et al) 1655 (Cambridge Univ. Press, 2014).
Seidov, D. Greenland–Iceland–Norwegian Seas Regional Climatology version 2 (Regional 497 Climatology Team, NOAA/NCEI, 2018).
Drange, H. & Simonsen, K. Formulation of Air–Sea Fluxes in the ESOP2 Version of MICOM (1996).
Acknowledgements
The authors received funding through the MARmaED project from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement number 675997. The results of this publication reflect only the authors’ view and the Commission is not responsible for any use that may be made of the information it contains. All authors received funding from the Research Council of Norway, project 294819.
Author information
Authors and Affiliations
Contributions
G.L., T.J.L. and C.J. conceived and designed the research. G.L. and T.J.L. developed the models with guidance from C.J. All the authors contributed to the interpretation of the results. G.L. wrote the paper with input from T.J.L. and C.J. The figures were created by T.J.L. with input from G.L. and C.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Mark Payne and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Model flowchart of drivers, mechanisms, and agents.
There are several commonalities between the Epipelagic and Mesopelagic fish model: both models base energy acquisition on visual encounters, which depend on light and prey availability, and physiological processes, including digestion and loss of energy, are temperature-dependent. Predation pressure is only included in the mesopelagic fish model.
Extended Data Fig. 2 Data localities and seasonal latitude distributions of zooplankton and temperature.
All simulations were run with seasonal light, idealised zooplankton prey fields (B), and temperature (C) at 30 m depth derived from data of current observations along a high latitude gradient from 55 to 75°N (A).
Extended Data Fig. 3 Current and future overwintering costs for the epipelagic planktivore.
The left panel shows the overwintering costs (kJ·ind−1·year−1) for current temperatures, the middle panel shows the change associated with a 2 °C warming, and the right panel shows the sum of panel 1 and 2, that is the new situation in an ocean that is 2 °C warmer. Values in left and right panel are normalized to the highest overwintering loss projected in the current scenario and isoclines represent the projected combination of latitude and body size for different percentages of these values. Red lines show the latitude that after 2 °C warming has equal overwintering cost as currently experienced by populations at 60°N and 70°N (blue dashed lines). For the highest latitudes (beyond ca. 74°N), the colder temperatures towards the poles reduce metabolic rates sufficiently to compensate for longer winters, making the total overwintering cost lower even though wintertime lasts longer. At the lowest latitudes (below ca. 58°N), the dark season is relatively short, but the smaller prey in the North Sea constrains energy intake in the largest individuals and makes winters comparatively favourable only for small fish.
Extended Data Fig. 4 Data localities and latitude-depth distributions of zooplankton and temperature.
All simulations were run with seasonal light, idealised zooplankton prey fields (B), and temperature (C) derived from data of current observations along a high latitude gradient from 55 to 75°N (A).
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, and methods M1–M4.
Supplementary Data 1
Source code for the epipelagic fish model.
Supplementary Data 2
Source code for the mesopelagic fish model.
Rights and permissions
About this article
Cite this article
Ljungström, G., Langbehn, T.J. & Jørgensen, C. Light and energetics at seasonal extremes limit poleward range shifts. Nat. Clim. Chang. 11, 530–536 (2021). https://doi.org/10.1038/s41558-021-01045-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-021-01045-2
This article is cited by
-
Adaptive photoperiod interpretation modulates phenological timing in Atlantic salmon
Scientific Reports (2023)
-
Incorporating mesopelagic fish into the evaluation of conservation areas for marine living resources under climate change scenarios
Marine Life Science & Technology (2023)
-
Diets of gadoid fish in Arctic waters of Svalbard fjords during the polar night
Polar Biology (2023)