Abstract
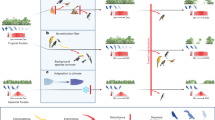
The resilience of biodiversity in the face of climate change depends on gene flow and range shifts. For diverse wind-dispersed and wind-pollinated organisms, regional wind patterns could either facilitate or hinder these movements, depending on alignment of winds with spatial climate patterns. We map global variation in terrestrial wind regimes, and model how ‘windscape’ connectivity will shape inbound and outbound dispersal between sites and their predicted future climate analogs. This model predicts that wind-accessible, climatically analogous sites will be scarcer in locations such as the tropics and on the leeward sides of mountain ranges, implying that the wind-dispersed biota in these landscapes may be more vulnerable to future climate change. A case study of Pinus contorta illustrates species-specific patterns of predicted genetic rescue and range expansion facilitated by wind. This framework has implications across fields ranging from historical biogeography and landscape genetics to ecological forecasting and conservation planning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the input data used in the study are publicly available. Source data that represent the results associated with each figure accompanies this paper.
Code availability
All R code used in the analysis has been deposited in the Zenodo data repository (https://doi.org/10.5281/zenodo.3860687)64, as has the source code for the version of the windscape R package developed and used for this study (https://doi.org/10.5281/zenodo.3857730)65.
References
Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W. & Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377 (2012).
Hampe, A. Plants on the move: the role of seed dispersal and initial population establishment for climate-driven range expansions. Acta Oecol. 37, 666–673 (2011).
Kremer, A. et al. Long‐distance gene flow and adaptation of forest trees to rapid climate change. Ecol. Lett. 15, 378–392 (2012).
Pecl, G. T. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214 (2017).
Felicísimo, Á. M., Muñoz, J. & González-Solis, J. Ocean surface winds drive dynamics of transoceanic aerial movements. PLoS ONE 3, e2928 (2008).
Gillespie, R. G. et al. Long-distance dispersal: a framework for hypothesis testing. Trends Ecol. Evol. 27, 47–56 (2012).
Muñoz, J., Felicísimo, Á. M., Cabezas, F., Burgaz, A. R. & Martínez, I. Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science 304, 1144–1147 (2004).
Smith, D. J. et al. Intercontinental dispersal of bacteria and archaea by transpacific winds. Appl. Environ. Microbiol. 79, 1134–1139 (2013).
Austerlitz, F., Dutech, C., Smouse, P. E., Davis, F. & Sork, V. L. Estimating anisotropic pollen dispersal: a case study in Quercus lobata. Heredity 99, 193–204 (2007).
Bullock, J. M. & Clarke, R. T. Long distance seed dispersal by wind: measuring and modelling the tail of the curve. Oecologia 124, 506–521 (2000).
Gassmann, M. I. & Pérez, C. F. Trajectories associated to regional and extra-regional pollen transport in the southeast of Buenos Aires province, Mar del Plata (Argentina). Int. J. Biometeorol. 50, 280–291 (2006).
Skarpaas, O. & Shea, K. Dispersal patterns, dispersal mechanisms, and invasion wave speeds for invasive thistles. Am. Naturalist 170, 421–430 (2007).
Wang, Z. F. et al. Pollen and seed flow under different predominant winds in wind-pollinated and wind-dispersed species Engelhardia roxburghiana. Tree Genet. Genomes 12, 19 (2016).
Soubeyrand, S., Enjalbert, J., Sanchez, A. & Sache, I. Anisotropy, in density and in distance, of the dispersal of yellow rust of wheat: experiments in large field plots and estimation. Phytopathology 97, 1315–1324 (2007).
Born, C., le Roux, P. C., Spohr, C., McGeoch, M. A. & van Vuuren, B. J. Plant dispersal in the sub‐Antarctic inferred from anisotropic genetic structure. Mol. Ecol. 21, 184–194 (2012).
Geremew, A., Woldemariam, M. G., Kefalew, A., Stiers, I. & Triest, L. Isotropic and anisotropic processes influence fine-scale spatial genetic structure of a keystone tropical plant. AoB Plants 10, plx076 (2018).
Brown, J. K. & Hovmøller, M. S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297, 537–541 (2002).
Vanschoenwinkel, B., Gielen, S., Seaman, M. & Brendonck, L. Any way the wind blows—frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos 117, 125–134 (2008).
Ahmed, S., Compton, S. G., Butlin, R. K. & Gilmartin, P. M. Wind-borne insects mediate directional pollen transfer between desert fig trees 160 kilometers apart. Proc. Natl Acad. Sci. USA 106, 20342–20347 (2009).
Larson-Johnson, K. Field observations of Carpinus (Betulaceae) demonstrate high dispersal asymmetry and inform migration simulations with implications for times of rapid climate change. Int. J. Plant Sci. 177, 389–399 (2016).
Nathan, R. et al. Spread of North American wind‐dispersed trees in future environments. Ecol. Lett. 14, 211–219 (2011).
Sorte, C. J. Predicting persistence in a changing climate: flow direction and limitations to redistribution. Oikos 122, 161–170 (2013).
Loarie, S. R. et al. The velocity of climate change. Nature 462, 1052–1055 (2009).
Molinos, J. G., Burrows, M. T. & Poloczanska, E. S. Ocean currents modify the coupling between climate change and biogeographical shifts. Sci. Rep. 7, 1332 (2017).
Higgins, S. I. et al. Forecasting plant migration rates: managing uncertainty for risk assessment. J. Ecol. 91, 341–347 (2003).
Bullock, J. M. et al. Modelling spread of British wind‐dispersed plants under future wind speeds in a changing climate. J. Ecol. 100, 104–115 (2012).
Kuparinen, A., Katul, G., Nathan, R. & Schurr, F. M. Increases in air temperature can promote wind-driven dispersal and spread of plants. Proc. R. Soc. B 276, 3081–3087 (2009).
Davis, H. G., Taylor, C. M., Lambrinos, J. G. & Strong, D. R. Pollen limitation causes an Allee effect in a wind-pollinated invasive grass (Spartina alterniflora). Proc. Natl Acad. Sci. USA 101, 13804–13807 (2004).
Dullinger, S., Dirnböck, T. & Grabherr, G. Patterns of shrub invasion into high mountain grasslands of the Northern Calcareous Alps, Austria. Arct. Antarct. Alp. Res. 35, 434–441 (2003).
Payette, S. The range limit of boreal tree species in Québec-Labrador: an ecological and palaeoecological interpretation. Rev. Palaeobot. Palynol. 79, 7–30 (1993).
Sandel, B., Monnet, A. C., Govaerts, R. & Vorontsova, M. Late Quaternary climate stability and the origins and future of global grass endemism. Ann. Bot. 119, 279–288 (2016).
Svenning, J. C. & Skov, F. Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol. Lett. 10, 453–460 (2007).
Schurr, F. M. et al. Colonization and persistence ability explain the extent to which plant species fill their potential range. Glob. Ecol. Biogeogr. 16, 449–459 (2007).
Saha, S. et al. The NCEP Climate Forecast System Reanalysis. Bull. Am. Meteorol. Soc. 91, 1015–1058 (2010).
Hamann, A., Roberts, D. R., Barber, Q. E., Carroll, C. & Nielsen, S. E. Velocity of climate change algorithms for guiding conservation and management. Glob. Change Biol. 21, 997–1004 (2015).
Kling, M. M., Auer, S. L., Comer, P. J., Ackerly, D. D. & Hamilton, H. Multiple axes of ecological vulnerability to climate change. Glob. Change Biol. 26, 2798–2813 (2020).
Keeley, A. T. et al. New concepts, models, and assessments of climate-wise connectivity. Environ. Res. Lett. 13, 073002 (2018).
Savage, D., Barbetti, M. J., MacLeod, W. J., Salam, M. U. & Renton, M. Timing of propagule release significantly alters the deposition area of resulting aerial dispersal. Diversity Distrib. 16, 288–299 (2010).
Nathan, R. et al. Long‐distance biological transport processes through the air: can nature’s complexity be unfolded in silico? Divers. Distrib. 11, 131–137 (2005).
Zeller, K. A., McGarigal, K. & Whiteley, A. R. Estimating landscape resistance to movement: a review. Landsc. Ecol. 27, 777–797 (2012).
Treml, E. A., Halpin, P. N., Urban, D. L. & Pratson, L. F. Modeling population connectivity by ocean currents, a graph-theoretic approach for marine conservation. Landsc. Ecol. 23, 19–36 (2008).
Fernández‐López, J. & Schliep, K. rWind: download, edit and include wind data in ecological and evolutionary analysis. Ecography 42, 804–810 (2019).
Thompson, S. & Katul, G. Plant propagation fronts and wind dispersal: an analytical model to upscale from seconds to decades using superstatistics. Am. Naturalist 171, 468–479 (2008).
Savage, D., Barbetti, M. J., MacLeod, W. J., Salam, M. U. & Renton, M. Can mechanistically parameterised, anisotropic dispersal kernels provide a reliable estimate of wind-assisted dispersal? Ecol. Model. 222, 1673–1682 (2011).
Regal, P. J. Pollination by wind and animals: ecology of geographic patterns. Annu. Rev. Ecol. Syst. 13, 497–524 (1982).
Carroll, C., Lawler, J. J., Roberts, D. R. & Hamann, A. Biotic and climatic velocity identify contrasting areas of vulnerability to climate change. PLoS ONE 10, e0140486 (2015).
Jackson, S. T. & Sax, D. F. Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends Ecol. Evol. 25, 153–160 (2010).
Ackerly, D. D. et al. The geography of climate change: implications for conservation biogeography. Divers. Distrib. 16, 476–487 (2010).
Owens, J. N. The Reproductive Biology of Lodgepole Pine Extension Note 07 (Forest Genetics Council of British Columbia, 2006).
Bontrager, M. & Angert, A. L. Gene flow improves fitness at a range edge under climate change. Evol. Lett. 3, 55–68 (2019).
Sexton, J. P., Strauss, S. Y. & Rice, K. J. Gene flow increases fitness at the warm edge of a species’ range. Proc. Natl Acad. Sci. USA 108, 11704–11709 (2011).
Rehfeldt, G. E., Ying, C. C., Spittlehouse, D. L. & Hamilton, D. A. Jr Genetic responses to climate in Pinus contorta: niche breadth, climate change, and reforestation. Ecol. Monogr. 69, 375–407 (1999).
Wang, T., O’Neill, G. A. & Aitken, S. N. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecol. Appl. 20, 153–163 (2010).
Karger, D. N. et al. Climatologies at high resolution for the Earth’s land surface areas. Sci. Data 4, 170122 (2017).
Dobrowski, S. Z. et al. The climate velocity of the contiguous United States during the 20th century. Glob. Change Biol. 19, 241–251 (2013).
van Etten, J. R Package gdistance: distances and routes on geographical grids. J. Stat. Softw. 76, 1–21 (2017).
IPCC Special Report on Global Warming of 1.5 °C (eds Masson-Delmotte, V. et al.) (WMO, 2018).
Schleussner, C. F. et al. Differential climate impacts for policy-relevant limits to global warming: the case of 1.5 °C and 2 °C. Earth Syst. Dyn. 7, 327–351 (2016).
Little, E. L. Jr Atlas of United States Trees. Volume 1, Conifers and Important Hardwoods Miscellaneous Publication 1146 (US Department of Agriculture, 1971).
Wang, T., Hamann, A., Yanchuk, A., O’Neill, G. A. & Aitken, S. N. Use of response functions in selecting lodgepole pine populations for future climates. Glob. Change Biol. 12, 2404–2416 (2006).
Phillips, S. J., Anderson, R. P. & Schapire, R. E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259 (2006).
Taylor, K. E., Stouffer, R. J. & Meehl, G. A. An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 93, 485–498 (2012).
R Core Team (2017). R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2017); https://www.R-project.org/
Kling, M. M. & Ackerly, D. D. Scripts and Data used in ‘Global Wind Patterns and the Vulnerability of Wind-Dispersed Species to Climate Change (Zenodo Repository, 2020); https://doi.org/10.5281/zenodo.3860687
Kling, M. M. Windscape R Package v.1.0.0 (Zenodo Repository, 2020); https://doi.org/10.5281/zenodo.3857730
Acknowledgements
M.K. was funded by a US National Science Foundation Graduate Research Fellowship. We thank B. Collins for providing access to wind data from CMIP5 simulations.
Author information
Authors and Affiliations
Contributions
M.K. conceived the study, conducted the analyses and drafted the manuscript. D.A. contributed discussion and feedback on the study design and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Gil Bohrer, Frank Schurr and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Global patterns of alignment between prevailing wind direction and temperature gradients.
a, Prevailing local wind direction, that is the bearing at which wind-dispersed organisms are expected to move on average. b, Direction of temperature gradient descent, that is the local direction in which organisms will need to move to offset warming climate. c, The difference between these two directions, with 0° indicating migratory tailwinds (prevailing winds blow directly down the temperature gradient) and 180° indicating migratory headwinds (prevailing winds blow directly up the temperature gradient).
Extended Data Fig. 2 Global patterns of landscape overlap between windsheds and climate analogs.
Maps show the amount of climatically analogous area versus the proportion of that area that is wind-accessible within 250 km of each focal site, in the outbound (a), and inbound (b), directions. (Panel a presents the same data as Fig. 4c of the main text, and is repeated here for comparison.) Color represents the bivariate relationship between these variables c, with green and blue indicating wind facilitation and yellow and red indicating wind hindrance. Additional scatterplots (d–f) compare the amount of similar climate, the amount of wind-accessible area, and the amount of wind-climate overlap in the forward versus reverse directions. Extreme outliers are rescaled in panel f for visual purposes only.
Extended Data Fig. 3 Global patterns of wind facilitation of climate change tracking.
Maps show wind facilitation for the landscape within 250km of each terrestrial grid cell, in the inbound (a), and outbound (b), directions, and with respect to major geographic gradients (c–e). In the scatterplots, latitude represents absolute latitude.
Extended Data Fig. 4 Global patterns of wind-climate overlap.
Maps show overlap for the landscape within 250km of each terrestrial grid cell, in the inbound (a), and outbound (b), directions, and with respect to major geographic gradients (c–e). In the scatterplots, latitude represents absolute latitude.
Extended Data Fig. 5 Global patterns of wind facilitation ‘syndromes’.
Sites can be assigned continuous rankings or discrete categories representing four alternative syndromes: wind facilitation, directional hindrance, speed hindrance, or climate limitation. a, Sites are ranked by climate availability, wind facilitation, and directional alignment (collapsed z-axis differentiating red from yellow) to assign relative membership in each of the four syndromes. b, Examples of each syndrome, with colors representing climate similarity, wind accessibility, and their areas of overlap across the 250 km radius landscapes surrounding each central origin cell. c,e, Syndrome prevalence by latitude in the inbound and outbound directions, respectively; syndromes are categorized to place 25% of global land area in each category, along the dotted lines depicted in panel a. d,f, Global map of syndromes in the inbound and outbound directions, respectively, with colors representing a continuous gradient among the four categories as depicted in panel a.
Extended Data Fig. 6 Global patterns of wind accessibility.
Maps show the mean wind accessibility of landscapes within 250km of each terrestrial grid cell, in the inbound (a), and outbound (b), directions, and with respect to major geographic gradients (c–e). In the scatterplots, latitude represents absolute latitude.
Extended Data Fig. 7 Global patterns of climate analog availability.
Maps show analog availability within 250km of each terrestrial grid cell, in the inbound (a), and outbound (b), directions, and with respect to major geographic gradients (c–e). In the scatterplots, latitude represents absolute latitude.
Supplementary information
Supplementary Information
Supplementary Discussion and Figs. 1–8.
Supplementary Data
Source data for Fig. 4 and Extended Data Figs. 2–4, 6 and 7 representing global patterns of windscape and climate change results.
Source data
Source Data Fig. 1
Spatial data for wind regimes.
Source Data Fig. 5
Spatial data for Pinus contorta results.
Source Data Extended Data Fig. 1
Spatial data for wind gradient alignment results.
Source Data Extended Data Fig. 5
Spatial data for wind facilitation syndrome results.
Rights and permissions
About this article
Cite this article
Kling, M.M., Ackerly, D.D. Global wind patterns and the vulnerability of wind-dispersed species to climate change. Nat. Clim. Chang. 10, 868–875 (2020). https://doi.org/10.1038/s41558-020-0848-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-0848-3
This article is cited by
-
Bioclimatic atlas of the terrestrial Arctic
Scientific Data (2023)
-
Potential distribution of fall armyworm in Africa and beyond, considering climate change and irrigation patterns
Scientific Reports (2022)
-
Compensation for wind drift during raptor migration improves with age through mortality selection
Nature Ecology & Evolution (2022)
-
Modelling influences of local and climatic factors on the occurrence and abundance of non-marine ostracods (Crustacea: Ostracoda) across Patagonia (Argentina)
Hydrobiologia (2022)
-
Far eastern curlew and whimbrel prefer flying low - wind support and good visibility appear only secondary factors in determining migratory flight altitude
Movement Ecology (2021)