Abstract
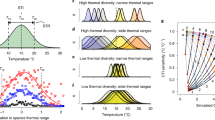
Marine ecosystems are under increasing threat from warming waters. Winter warming is occurring at a faster rate than summer warming for ecosystems around the world, but most studies focus on the summer. Here, we show that winter warming could affect coastal fish community compositions in the Mediterranean Sea using a model that captures how biotic associations change with sea surface temperature to influence species’ distributions for 215 fish species. Species’ associations control how communities are formed, but the effect of winter warming on associations will be on average four times greater than that of summer warming. Projections using future climate scenarios show that 60% of coastal Mediterranean grid cells are expected to lose fish species by 2040. Heavily fished areas in the west will experience diversity losses that exacerbate regime shifts linked to overexploitation. Incorporating seasonal differences will therefore be critical for developing effective coastal fishery and marine ecosystem management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The Mediterranean fish binary occurrence data and IPCC SRES A2 SST projections that support the findings of this study are described in ref. 31 and are available in the Ecological Archives (accession E096-203-D1).
Code availability
All R code needed to extract data from public repositories, replicate all of the analyses and generate the figures is presented in the Supplementary Information file and stored in a licensed GitHub repository (https://github.com/nicholasjclark/Mediterranean-Fishes-MRF).
References
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (2005).
Albouy, C. et al. Projected climate change and the changing biogeography of coastal Mediterranean fishes. J. Biogeogr. 40, 534–547 (2013).
Hoegh-Guldberg, O. et al. Impacts of 1.5 °C global warming on natural and human systems. in Special Report on Global Warming of 1.5 °C (eds Masson-Delmotte, V. et al.) 212–251 (WMO, 2018).
Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018).
Smale, D. A. et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change 9, 306–312 (2019).
EUROSTAT EU Fishery Economic Report 2010. European Union Mediterranean and Black Sea Fishing Fleet (FIRMS, 2010).
Abulafia, D. The Great Sea: a Human History of the Mediterranean (Oxford Univ. Press, 2011).
Giakoumi, S. et al. Ecoregion-based conservation planning in the Mediterranean: dealing with large-scale heterogeneity. PLoS ONE 8, e76449 (2013).
Katsanevakis, S. et al. Invading the Mediterranean Sea: biodiversity patterns shaped by human activities. Front. Mar. Sci. 1, 32 (2014).
Pinardi, N., Arneri, E., Crise, A., Ravaioli, M. & Zavatarelli, M. in The Sea Vol. 14 (eds Robinson, A. & Brink, K.) 1243–1330 (Harvard Univ. Press, 2006).
Lejeusne, C., Chevaldonné, P., Pergent-Martini, C., Boudouresque, C. F. & Perez, T. Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 25, 250–260 (2010).
Cramer, W. et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Change 8, 972–980 (2018).
Bintanja, R. & van der Linden, E. C. The changing seasonal climate in the Arctic. Sci. Rep. 3, 1556 (2013).
Kharin, V. V., Zwiers, F. W., Zhang, X. & Wehner, M. Changes in temperature and precipitation extremes in the CMIP5 ensemble. Clim. Change 119, 345–357 (2013).
Thibeault, J. M. & Seth, A. Changing climate extremes in the Northeast United States: observations and projections from CMIP5. Clim. Change 127, 273–287 (2014).
Both, C. et al. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. Lond. B Biol. Sci. 2010, 1259–1266 (1685).
Chesson, P. et al. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141, 236–253 (2004).
Tonkin, J. D., Bogan, M. T., Bonada, N., Rios-Touma, B. & Lytle, D. A. Seasonality and predictability shape temporal species diversity. Ecology 98, 1201–1216 (2017).
Hiddink, J. G. & Ter Hofstede, R. Climate induced increases in species richness of marine fishes. Glob. Change Biol. 14, 453–460 (2008).
Rutterford, L. A. et al. Future fish distributions constrained by depth in warming seas. Nat. Clim. Change 5, 569–573 (2015).
Colloca, F., Scarcella, G. & Libralato, S. Recent trends and impacts of fisheries exploitation on Mediterranean stocks and ecosystems. Front. Mar. Sci. 4, 244 (2017).
Barange, M. et al. Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options. FAO Fisheries and Aquaculture Technical Paper No. 627 (FAO, 2018).
IPCC Climate Change 2014: Synthesis Report (eds Core Writing Team, Pachauri, R. K. & Meyer, L. A.) (IPCC, 2014).
Rice, J. C. & Garcia, S. M. Fisheries, food security, climate change, and biodiversity: characteristics of the sector and perspectives on emerging issues. ICES J. Mar. Sci. 68, 1343–1353 (2011).
Free, C. M. et al. Impacts of historical warming on marine fisheries production. Science 363, 979–983 (2019).
Ben Rais Lasram, F. et al. The Mediterranean Sea as a ‘cul‐de‐sac’ for endemic fishes facing climate change. Glob. Change Biol. 16, 3233–3245 (2010).
Yeager, L. A., Deith, M. C., McPherson, J. M., Williams, I. D. & Baum, J. K. Scale dependence of environmental controls on the functional diversity of coral reef fish communities. Glob. Ecol. Biogeogr. 26, 1177–1189 (2017).
Zenetos, A. et al. Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. Mediterr. Mar. Sci. 6, 63–118 (2005).
The State of Mediterranean and Black Sea Fisheries: 2018 (FAO & General Fisheries Commission for the Mediterranean, 2018).
Scientific Technical and Economic Committee for Fisheries (STECF) The 2015 Annual Economic Report on the EU Fishing Fleet (STECF-15-07) (Publications Office of the European Union, 2015).
Albouy, C. et al. FishMed: traits, phylogeny, current and projected species distribution of Mediterranean fishes, and environmental data. Ecology 96, 2312–2313 (2015).
Beuvier, J. et al. Modeling the Mediterranean Sea interannual variability during 1961–2000: focus on the Eastern Mediterranean Transient. J. Geophys. Res. Oceans 115, C08017 (2010).
Arnell, N. W. Climate change and global water resources: SRES emissions and socio-economic scenarios. Glob. Environ. Change 14, 31–52 (2004).
Adloff, F. et al. Mediterranean Sea response to climate change in an ensemble of twenty first century scenarios. Clim. Dynam. 45, 2775–2802 (2015).
Whitehead, P. J. P., Bauchot, M., Hureau, J., Nielsen, J. & Tortonese, E. Fishes of the North-Eastern Atlantic and the Mediterranean Vol. 1 (UNESCO, 1984).
Gonçalves, A. R., Von Zuben, F. J. & Banerjee, A. Multi-label structure learning with Ising model selection. In Proc. Twenty-Fourth International Joint Conference on Artificial Intelligence 3525–3531 (AAAI Press, 2015).
Galar, M., Fernandez, A., Barrenechea, E., Bustince, H. & Herrera, F. A review on ensembles for the class imbalance problem: bagging-, boosting-, and hybrid-based approaches. IEEE Trans. Syst. Man. Cybern. C Appl. Rev. 42, 463–484 (2012).
Gravel, D. et al. Bringing Elton and Grinnell together: a quantitative framework to represent the biogeography of ecological interaction networks. Ecography 42, 401–415 (2019).
Dayton, P. K. Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol. Monogr. 41, 351–389 (1971).
Dormann, C. F. et al. Biotic interactions in species distribution modelling: 10 questions to guide interpretation and avoid false conclusions. Glob. Ecol. Biogeogr. 27, 1004–1016 (2018).
Dormann, C. F., Fründ, J. & Schaefer, H. M. Identifying causes of patterns in ecological networks: opportunities and limitations. Annu. Rev. Ecol. Evol. Syst. 48, 559–584 (2017).
Golding, N., Nunn, M. A. & Purse, B. V. Identifying biotic interactions which drive the spatial distribution of a mosquito community. Parasites Vectors 8, 367 (2015).
Harris, D. J. Generating realistic assemblages with a joint species distribution model. Methods Ecol. Evol. 6, 465–473 (2015).
Thorson, J. T. et al. Joint dynamic species distribution models: a tool for community ordination and spatio-temporal monitoring. Glob. Ecol. Biog. 25, 1144–1158 (2016).
Azaele, S., Muneepeerakul, R., Rinaldo, A. & Rodriguez-Iturbe, I. Inferring plant ecosystem organization from species occurrences. J. Theor. Biol. 262, 323–329 (2010).
Harris, D. J. Inferring species interactions from co‐occurrence data with Markov networks. Ecology 97, 3308–3314 (2016).
Cheng, J., Levina, E., Wang, P. & Zhu, J. A sparse Ising model with covariates. Biometrics 70, 943–953 (2014).
Lindberg, O. Markov Random Fields in Cancer Mutation Dependencies. MSc Thesis, Univ. Turku (2016).
Clark, N. J., Wells, K. & Lindberg, O. Unravelling changing interspecific interactions across environmental gradients using Markov random fields. Ecology 99, 1277–1283 (2018).
Lee, J. D. & Hastie, T. J. Learning the structure of mixed graphical models. J. Comput. Graph. Stat. 24, 230–253 (2015).
Wood, S. N. Thin plate regression splines. J. R. Stat. Soc. B Stat. Methodol. 65, 95–114 (2003).
Kammann, E. & Wand, M. P. Geoadditive models. J. R. Stat. Soc. C Appl. Stat. 52, 1–18 (2003).
McInerny, G. J. & Purves, D. W. Fine‐scale environmental variation in species distribution modelling: regression dilution, latent variables and neighbourly advice. Methods Ecol. Evol. 2, 248–257 (2011).
Givan, O., Parravicini, V., Kulbicki, M. & Belmaker, J. Trait structure reveals the processes underlying fish establishment in the Mediterranean. Glob. Ecol. Biogeogr. 26, 142–153 (2017).
Mouillot, D. et al. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc. Natl Acad. Sci. USA 111, 13757–13762 (2014).
Tsirogiannis, C. & Sandel, B. PhyloMeasures: a package for computing phylogenetic biodiversity measures and their statistical moments. Ecography 39, 709–714 (2015).
Webb, C. O., Ackerly, D. D. & Kembel, S. W. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24, 2098–2100 (2008).
Newman, M. E. Modularity and community structure in networks. Proc. Natl Acad. Sci. USA 103, 8577–8582 (2006).
Csárdi, G. & Nepusz, T. The igraph software package for complex network research. InterJournal Complex Syst. 1965, 1–9 (2006).
Pellissier, L. et al. Comparing species interaction networks along environmental gradients. Biol. Rev. 93, 785–800 (2017).
Miller, P. J., Lubke, G. H., McArtor, D. B. & Bergeman, C. S. Finding structure in data using multivariate tree boosting. Psychol. Methods 21, 583–602 (2016).
R Development Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2017).
Clark, N. J., Wells, K. & Lindberg, O. MRFcov: Markov random fields with additional covariates. R package version 1.0 https://github.com/nicholasjclark/MRFcov (2018).
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B Stat. Methodol. 73, 3–36 (2011).
Wickham, H. & Francois, R. dplyr: A grammar of data manipulation. R package version 0.7.2 https://CRAN.R-project.org/package=dplyr (2017).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
Acknowledgements
We thank the creators and maintainers of the FishMed database for facilitating access to occurrence and IPCC projection data. The co-occurrence modelling was undertaken on facilities at the Research Computing Centre at the University of Queensland, which is supported by the Australian Commonwealth Government. N.J.C. is supported by a University of Queensland Early Career Research Grant (UQECR1946913). C.I.F. is supported by a Rutherford Discovery Fellowship from the Royal Society of New Zealand (UOO1803).
Author information
Authors and Affiliations
Contributions
N.J.C. conceived of the idea, conducted the analyses and wrote most of the first draft of the paper. J.T.K. and C.I.F. contributed conceptual advice and helped with writing the paper and with refining later drafts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Sandro Azaele, Marta Coll and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Schematic overview of the ensemble modelling approach and examples of key outputs produced at each step of analysis.
Schematic overview of the ensemble modelling approach and examples of key outputs produced at each step of analysis. A spatial Conditional Random Fields (CRF) model was trained on 1980 binary occurrence vectors for 215 fish species across 8,154 coastal sample sites in the Mediterranean Sea, using mean summer and mean winter Sea Surface Temperatures as external predictors. To generate predictions for a range of climate scenarios, simulation from the CRFs posterior predictive distribution was accomplished using a multivariate boosted regression tree that learned complex, nonlinear relationships and prioritised those predictors that had large influences on covariance in species’ occurrence probabilities. These simulations allowed for more direct comparisons among historical and future predictions, avoiding the biases that can occur when comparing observed and predicted community measurements.
Extended Data Fig. 2 Predicted historical and future metrics of fish community species richness, functional diversity and network modularity across coastal grid cells in the Mediterranean Sea.
Predicted historical (1980) and future (2040) metrics of fish community species richness, functional diversity and network modularity across coastal grid cells in the Mediterranean Sea. Predictions were based on sea surface temperate (SST) estimates using IPCC SRES A2 climate scenarios. Note that functional diversity and network modularity metrics are unitless and are therefore presented as standardised estimates where very low: ≤ 7.5 percentile; low: 7.5 – 37.5 percentile; moderate: 37.5 – 62.5 percentile; high: 62.5 – 92.5 percentile; very high: ≥ 92.5 percentile.
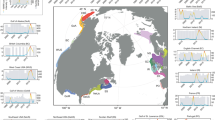
Extended Data Fig. 3 Geographical distributions of coastal fishing zones and populous coastal cities in the Mediterranean Sea.
Geographical distributions of coastal fishing zones (Geographical Subareas; GSAs) and populous coastal cities in the Mediterranean Sea. Regions highlighted in colour correspond to key geographical areas that are expected to experience marked changes in their coastal fish communities in response to warming sea surface temperatures.
Extended Data Fig. 4 Relationships between winter warming classification and predicted changes in coastal fish community richness, functional diversity and network modularity between 1980 and 2040 IPCC SRES A2 climate scenarios.
(a) Coastal sample sites in the Mediterranean Sea classified according to whether a grid cell is predicted to surpass a 13 °C winter sea surface temperature (SST) threshold by the year 2040. (b) Relationships between winter warming classification and predicted changes in coastal fish community richness, functional diversity and network modularity between 1980 and 2040 IPCC SRES A2 climate scenarios. Boxplots show: medians (lines within boxes), 25% and 75% quantiles (hinges) and 5% and 95% quantiles (whiskers).
Extended Data Fig. 5 Average predicted changes in coastal fish species community richness, functional diversity and network modularity across GSAs between 1980 and 2040 IPCC SRES A2 climate scenarios.
(a, b, c) Geographical variation in fishing pressures across Mediterranean Sea GSAs, calculated as total landings per km2 of coastal shelf area, for total fishes, demersal species and pelagic species. (d, e, f) Average predicted changes in coastal fish species community richness, functional diversity and network modularity across GSAs between 1980 and 2040 IPCC SRES A2 climate scenarios.
Extended Data Fig. 6 Predicted latitudinal distributions in the 1980 and 2040 time periods for species of economic and conservation importance.
Predicted latitudinal distributions in the 1980 and 2040 time periods for species of economic and conservation importance, including non-indigenous species with the greatest potential impacts (according to the General Fisheries Commission for the Mediterranean). Range sizes were calculated by summing the predicted presence / absence vectors in each time period across all 8,154 grid cells.
Extended Data Fig. 7 Predicted longitudinal distributions in the 1980 and 2040 time periods for species of economic and conservation importance.
Predicted longitudinal distributions in the 1980 and 2040 time periods for species of economic and conservation importance, including non-indigenous species with the greatest potential impacts (according to the General Fisheries Commission for the Mediterranean). Range sizes were calculated by summing the predicted presence / absence vectors in each time period across all 8,154 grid cells.
Supplementary information
Supplementary Information
Supplementary Tables 1–3 and Appendices 1–3.
Rights and permissions
About this article
Cite this article
Clark, N.J., Kerry, J.T. & Fraser, C.I. Rapid winter warming could disrupt coastal marine fish community structure. Nat. Clim. Chang. 10, 862–867 (2020). https://doi.org/10.1038/s41558-020-0838-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-0838-5
This article is cited by
-
Future climate projections for Eastern Canada
Climate Dynamics (2022)