Abstract
There is broad consensus that, via changes in stomatal conductance, plants moderate the exchanges of water and carbon between the biosphere and atmosphere, playing a major role in global hydroclimate. Tree rings record atmospheric CO2 concentration (ca) and its isotopic composition (13C/12C)—mediated by stomatal and photosynthetic influences—that can be expressed in terms of intrinsic water-use efficiency (W). Here, we compile a global W dataset based on 422 tree-ring isotope series and report that W increased with ca over the twentieth century, but the rates of increase (dW/dca) declined by half. Angiosperms contributed more than gymnosperms to the slowdown, and in recent decades, dW/dca for angiosperms was close to zero. dW/dca varies widely across climatic regions and reflects pauses in emissions during the Great Depression and after World War II. There is strong spatial variability in climate forcing via an increasing W, which is weakening globally with time.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data for W can be accessed via the Zenodo repository (https://doi.org/10.5281/zenodo.3693240). Source data for Fig. 1 are provided with the paper.
Code availability
The code used in processing data can be accessed via the Zenodo repository (https://doi.org/10.5281/zenodo.3693240).
References
Peters, W. et al. Increased water-use efficiency and reduced CO2 uptake by plants during droughts at a continental scale. Nat. Geosci. 11, 744–748 (2018).
Skinner, C. B. et al. The role of plant CO2 physiological forcing in shaping future daily-scale precipitation. J. Clim. 30, 2319–2340 (2017).
Frank, D. C. et al. Water-use efficiency and transpiration across European forests during the Anthropocene. Nat. Clim. Change 5, 579–583 (2015).
van der Sleen, P. et al. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 8, 24–28 (2015).
Marvel, K. et al. Twentieth-century hydroclimate changes consistent with human influence. Nature 569, 59–65 (2019).
Betts, R. A. et al. Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature 448, 1037–1041 (2007).
Kooperman, G. J. et al. Forest response to rising CO2 drives zonally asymmetric rainfall change over tropical land. Nat Clim. Change 8, 434–440 (2018).
Richardson, T. B. et al. Carbon dioxide physiological forcing dominates projected eastern Amazonian drying. Geophys. Res. Lett. 45, 2815–282 (2018).
Langenbrunner, B., Pritchard, M. S., Kooperman, G. J. & Randerson, J. T. Why does Amazon precipitation decrease when tropical forests respond to increasing CO2? Earths Future 7, 450–468 (2018).
Keeling, R. F. et al. Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis. Proc. Natl Acad. Sci. USA 114, 10361–10366 (2017).
Franks, P. J. et al. Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century. New Phytol. 197, 1077–1094 (2013).
Keenan, T. F. et al. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499, 324–327 (2013).
Norby, R. J. & Zak, D. R. Ecological lessons from free-air CO2 enrichment (FACE) experiments. Ann. Rev. Ecol. Evol. Syst. 42, 181–203 (2011).
Cao, L. et al. Importance of carbon dioxide physiological forcing to future climate change. Proc. Natl Acad. Sci. USA 107, 9513–9518 (2010).
de Boer, H. J. et al. Climate forcing due to optimization of maximal leaf conductance in subtropical vegetation under rising CO2. Proc. Natl Acad. Sci. USA 108, 4041–4046 (2011).
Richardson, T. B., Forster, P. M., Andrews, T. & Parker, D. J. Understanding the rapid precipitation response to CO2 and aerosol forcing on a regional scale. J. Clim. 29, 583–594 (2016).
Skinner, C. B., Poulsen, C. J. & Mankin, J. S. Amplification of heat extremes by plant CO2 physiological forcing. Nat. Commun. 9, 1094 (2018).
Kala, J. et al. Impact of the representation of stomatal conductance on model projections of heatwave intensity. Sci. Rep. 6, 23418 (2016).
Adams, M. A., Buckley, T. N. & Turnbull, T. L. Rainfall drives variation in rates of change in intrinsic water use efficiency of tropical forests. Nat. Commun. 10, 3661 (2019).
Estrada, F., Perron, P. & Martinez-López, B. Statistically derived contributions of diverse human influences to twentieth-century temperature changes. Nat. Geosci. 6, 1050–1055 (2013).
Bastos, A. et al. Re-evaluating the 1940s CO2 plateau. Biogeosci. 13, 4877–4897 (2016).
Hofmann, D., Butler, J. H. & Tans, P. P. A new look at atmospheric carbon dioxide. Atmos. Environ. 43, 2084–2086 (2009).
Ainsworth, E. A. & Long, S. P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372 (2005).
Oren, R. et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 41, 469–472 (2001).
Guerrieri, R. et al. Disentangling the role of photosynthesis and stomatal conductance on rising forest water-use efficiency. Proc. Natl Acad. Sci. USA 116, 16909–16914 (2019).
Lin, Y.-S. et al. Optimal stomatal behaviour around the world. Nat. Clim. Change 5, 459–464 (2015).
Klein, T. & Ramon, U. Stomatal sensitivity to CO2 diverges between angiosperm and gymnosperm tree species. Funct. Ecol. 33, 1411–1424 (2019).
Craine, J. M. et al. Isotopic evidence for oligotrophication of terrestrial ecosystems. Nat. Ecol. Evol. 2, 1735–1744 (2018).
Donohue, R. J., Roderick, M. L., McVicar, T. R. & Yang, Y. A simple hypothesis of how leaf and canopy-level transpiration and assimilation respond to elevated CO2 reveals distinct response patterns between disturbed and undisturbed vegetation. J. Geophys. Res. Biogeosci. 122, 168–18415 (2017).
Yang, Y. et al. Contrasting responses of water use efficiency to drought across global terrestrial ecosystems. Sci. Rep. 6, 23284 (2016).
Tang, X. et al. How is water-use efficiency of terrestrial ecosystems distributed and changing on Earth? Sci. Rep. 4, 7483 (2014).
Esper, J. et al. Low-frequency noise in δ13C and δ18O tree ring data: a case study of Pinus uncinata in the Spanish Pyrenees. Glob. Biogeochem. Cycles 24, GB4018 (2010).
Jasechko, S. et al. Terrestrial water fluxes dominated by transpiration. Nature 496, 347–350 (2012).
McCarroll, D. & Loader, N. J. Stable isotopes in tree rings. Q. Sci. Rev. 23, 771–801 (2004).
Robertson, A. et al. Hypothesized climate forcing time series for the last 500 years. J. Geophys. Res. 106, 14783–14803 (2001).
Keeling, C. D. et al. in A History of Atmospheric CO 2 and its effects on Plants, Animals and Ecosystems (eds Ehleringer, J. R. et al.) 83–113 (Springer, 2005).
Ubierna, N. Holloway-Phillips, M.-M. & Farquhar, G. D. in Photosynthesis—Methods and Protocols (Ed. Covshoff, S.) 155–196 (Humana Press, 2018).
Farquhar, G. D. et al. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Wong, S. C. et al. Stomatal conductance correlates with photosynthetic capacity. Nature 282, 424–426 (1979).
De Pury, D. G. G. & Farquhar, G. D. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ. 20, 537–557 (1997).
Brienen, R. J. W. et al. Tree height strongly affects estimates of water-use efficiency responses to climate and CO2 using isotopes. Nat. Commun. 8, 288 (2017).
Loader, N. J. et al. Stable carbon isotopes from Torneträsk, northern Sweden provide a millennial length reconstruction of summer sunshine and its relationship to Arctic circulation. Q. Sci. Rev. 62, 97–113 (2013).
Acknowledgements
We thank the authors who provided additional information that allowed us to include their data in this work. We thank A. Barlow for help with digitizing the isotope series and M. Gharun for help with climate data. M.A.A. and T.L.T. thank the Australian Research Council for general support. M.A.A. thanks an anonymous donor for funding of this study. T.N.B. acknowledges support from the National Science Foundation (grant no. 1557906) and the USDA National Institute of Food and Agriculture (Hatch project 1016439).
Author information
Authors and Affiliations
Contributions
M.A.A., T.L.T. and T.N.B. developed the original ideas included in this paper. T.L.T. screened the literature, led the extraction of data and characterized the dataset. T.N.B. interrogated the dataset for analysis and prepared the modelling. T.L.T. analysed the data and prepared the figures and tables. M.A.A. wrote the paper with T.L.T. and T.N.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Katrin Fleischer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Relationship of W to sample year.
For all 422 tree-ring isotope series containing data from the period 1851–2015, W was calculated using Equation 3.
Extended Data Fig. 2 Effects of methods of calculation of W.
a, Relationship between dW/dca calculated using Equation 2 with that calculated using Equation 3 (based on 13C/12C ratios of wood tissue for all series shown in Extended Data Fig. 1. b, Example of the difference in calculated W if either Equations 2 or 3 are applied to isotope data as extracted from Loader et al.42.
Extended Data Fig. 3 Descriptive statistics for the global dataset of rates of change in W with ca (dW/dca).
Data are as shown for Extended Data Fig. 1. a, Frequency of dW/dca. b, Frequency of R2 values for relationships between W and ca. (c) Frequency of P-values for relationships between W and ca.
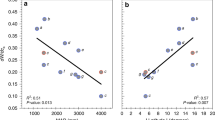
Extended Data Fig. 4 Effects of climatic variables on rates of change in W with ca (dW/dca).
Linear regressions of dW/dca and climatic variables: mean annual precipitation, MAP; potential evapotranspiration, PET; mean annual temperature, MAT; vapour pressure; and radiation. (a–e) All data as shown in Extended Data Fig. 1. (f–o) Angiosperms (n = 147) and gymnosperms (n = 275) considered separately.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and references (data sources).
Rights and permissions
About this article
Cite this article
Adams, M.A., Buckley, T.N. & Turnbull, T.L. Diminishing CO2-driven gains in water-use efficiency of global forests. Nat. Clim. Chang. 10, 466–471 (2020). https://doi.org/10.1038/s41558-020-0747-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-0747-7
This article is cited by
-
Modeling the effect of grazing on carbon and water use efficiencies in grasslands on the Qinghai–Tibet Plateau
BMC Ecology and Evolution (2024)
-
Carboxylate-releasing Eucalyptus patens in a low-diversity spot in a biodiversity hotspot in Southwest Australia is not a ‘Darwinian demon’
Plant and Soil (2024)
-
Decline in tree-ring growth of Picea mongolica and its intra-annual eco-physiological responses to drought and CO2 enrichment in semi-arid China
Journal of Forestry Research (2024)
-
Rising water-use efficiency in European grasslands is driven by increased primary production
Communications Earth & Environment (2023)
-
Increased photosynthesis during spring drought in energy-limited ecosystems
Nature Communications (2023)