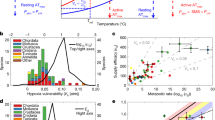
Abstract
Rapid climate change is postulated to cause marine extinctions, especially among climate-sensitive clades, traits and regions1,2,3,4,5,6. This premise is based on two hypotheses: (1) known individual physiological sensitivities scale up to macroecological selectivity patterns4,7,8 and (2) ancient hyperthermal events are appropriate models to anticipate ecological winners and losers of anthropogenic climate change9. Yet these hypotheses have largely escaped quantitative appraisal. Here we show that experimental responses of modern marine ectotherms to single and combined climate-related stressors (such as seawater warming, hypoxia and acidification) align with Phanerozoic fossil extinction regimes across clades and functional traits. Of climate-related stressors, the synergistic interaction between warming and hypoxia10, encumbering aerobic metabolism, has the greatest potency as a proximate driver of extinction. All else being equal8, this synergy particularly imperils modern warm-water organisms. Modern–fossil agreement is strongest at intermediate–high extinction intensities and hyperthermal events but may fail at extreme extinction events, perhaps due to rising prominences of, and interactions among, additional biotic and abiotic stressors. According to results from marine ectotherms, clade-based sensitivity of individuals to climate-related stressors scales up from subannual experiments and decadal range-shift response magnitudes11, to extinction selectivity patterns at ancient climate-related stressor events and the Phanerozoic durations of genera.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Fossil occurrence data are available from the Paleobiology Database (http://www.paleobiodb.org) and data on thermal ranges of species are available from Aquamaps (http://www.aquamaps.org). Raw data required to replicate the analysis are accessible via Zenodo at https://doi.org/10.5281/zenodo.3465281.
Code availability
Scripts required to replicate the analysis are accessible via Zenodo at https://doi.org/10.5281/zenodo.3465281. The basic data and main figures themselves can be reproduced using these scripts, as well as Extended Data Figs. 1, 4, 6 and 10.
References
Harnik, P. G. et al. Extinction in ancient and modern seas. Trends Ecol. Evol. 27, 608–617 (2012).
Finnegan, S. et al. Paleontological baselines for evaluating extinction risk in the modern oceans. Science 348, 567–570 (2015).
Kiessling, W. & Simpson, C. On the potential for ocean acidification to be a general cause of ancient reef crises. Glob. Change Biol. 17, 56–67 (2011).
Knoll, A. H., Bambach, R. K., Payne, J. L., Pruss, S. & Fischer, W. W. Paleophysiology and end-Permian mass extinction. Earth Planet. Sci. Lett. 256, 295–313 (2007).
Reddin, C. J., Kocsis, Á. T. & Kiessling, W. Climate change and the latitudinal selectivity of ancient marine extinctions. Paleobiology 45, 70–84 (2019).
García Molinos, J. et al. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change 6, 83–88 (2016).
Calosi, P., Putnam, H. M., Twitchett, R. J. & Vermandele, F. Marine metazoan modern mass extinction: improving predictions by integrating fossil, modern, and physiological data. Annu. Rev. Mar. Sci. 11, 13–25 (2019).
Penn, J. L., Deutsch, C., Payne, J. L. & Sperling, E. A. Temperature-dependent hypoxia explains biogeography and severity of end-Permian marine mass extinction. Science 362, eaat1327 (2018).
Foster, G. L., Hull, P., Lunt, D. J. & Zachos, J. C. Placing our current ‘hyperthermal’ in the context of rapid climate change in our geological past. Phil. Trans. R. Soc. A 376, 20170086 (2018).
Vaquer-Sunyer, R. & Duarte, C. M. Temperature effects on oxygen thresholds for hypoxia in marine benthic organisms. Glob. Change Biol. 17, 1788–1797 (2011).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925 (2013).
Breitburg, D. et al. Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240 (2018).
Cahill, A. E. et al. How does climate change cause extinction?. Proc. R. Soc. B 280, 20121890 (2013).
Kemp, D. B., Eichenseer, K. & Kiessling, W. Maximum rates of climate change are systematically underestimated in the geological record. Nat. Commun. 6, 8890 (2015).
Wittmann, A. C. & Pörtner, H. O. Sensitivities of extant animal taxa to ocean acidification. Nat. Clim. Change 3, 995–1001 (2013).
Storch, D., Menzel, L., Frickenhaus, S. & Pörtner, H. O. Climate sensitivity across marine domains of life: limits to evolutionary adaptation shape species interactions. Glob. Change Biol. 20, 3059–3067 (2014).
Harvey, B. P., Gwynn-Jones, D. & Moore, P. J. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 3, 1016–1030 (2013).
Kroeker, K. J. et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896 (2013).
Przeslawski, R., Byrne, M. & Mellin, C. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Change Biol. 21, 2122–2140 (2015).
Darling, E. S. & Côté, I. M. Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286 (2008).
Pörtner, H.-O., Bock, C. & Mark, F. C. Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J. Exp. Biol. 220, 2685–2696 (2017).
Deutsch, C., Ferrel, A., Seibel, B., Pörtner, H.-O. & Huey, R. Climate change tightens a metabolic constraint on marine habitats. Science 348, 1132–1136 (2015).
Kroeker, K. J., Kordas, R. L. & Harley, C. D. G. Embracing interactions in ocean acidification research: confronting multiple stressor scenarios and context dependence. Biol. Lett. 13, 20160802 (2017).
Tewksbury, J. J., Huey, R. B. & Deutsch, C. A. Putting the heat on tropical animals. Science 320, 1296–1297 (2008).
Rohr, J. R. et al. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 21, 1425–1439 (2018).
Saupe, E. E. et al. Macroevolutionary consequences of profound climate change on niche evolution in marine molluscs over the past three million years. Proc. R. Soc. B 281, 20141995 (2014).
Hopkins, M. J., Simpson, C. & Kiessling, W. Differential niche dynamics among major marine invertebrate clades. Ecol. Lett. 17, 314–323 (2014).
Peters, S. E. Environmental determinants of extinction selectivity in the fossil record. Nature 454, 626–629 (2008).
Schoene, B., Guex, J., Bartolini, A., Schaltegger, U. & Blackburn, T. J. Correlating the end-Triassic mass extinction and flood basalt volcanism at the 100 ka level. Geology 38, 387–390 (2010).
Jablonski, D. Mass extinctions and macroevolution. Paleobiology 31, 192–210 (2005).
Durack, P. J., Wijffels, S. E. & Matear, R. J. Ocean salinities reveal strong global water cycle intensification during 1950 to 2000. Science 336, 455–459 (2012).
Hendriks, I. E., Duarte, C. M. & Álvarez, M. Vulnerability of marine biodiversity to ocean acidification: a meta-analysis. Estuar. Coast. Shelf Sci. 86, 157–164 (2010).
Wernberg, T., Smale, D. A. & Thomsen, M. S. A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob. Change Biol. 18, 1491–1498 (2012).
Crain, C. M., Kroeker, K. & Halpern, B. S. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315 (2008).
Tummers, B. DataThief III v.1.7 (2006); https://datathief.org
Pörtner, H. O. & Farrell, A. P. Physiology and climate change. Science 322, 690–692 (2008).
Woods, H. A., Harrison, J. F. & Merila, J. Interpreting rejections of the beneficial acclimation hypothesis: when is physiological plasticity adaptive? Evolution 56, 1863–1866 (2002).
Boag, T. H., Stockey, R. G., Elder, L. E., Hull, P. M. & Sperling, E. A. Oxygen, temperature and the deep-marine stenothermal cradle of Ediacaran evolution. Proc. R. Soc. B 285, 20181724 (2018).
Kaschner, K. et al. AquaMaps: predicted range maps for aquatic species v.08/2016 (2016); www.aquamaps.org
Valentine, J. W. Temporal bias in extinctions among taxonomic categories. J. Paleontol. 48, 549–552 (1974).
Bambach, R. K., Knoll, A. H. & Sepkoski, J. J. Anatomical and ecological constraints on Phanerozoic animal diversity in the marine realm. Proc. Natl Acad. Sci. USA 99, 6854–6859 (2002).
Hönisch, B. et al. The geological record of ocean acidification. Science 335, 1058–1063 (2012).
Harrison, F. Getting started with meta-analysis. Methods Ecol. Evol. 2, 1–10 (2011).
Gurevitch, J., Koricheva, J., Nakagawa, S. & Stewart, G. Meta-analysis and the science of research synthesis. Nature 555, 175–182 (2018).
Gurevitch, J., Morrison, J. A. & Hedges, L. V. The Interaction between competition and predation: a meta‐analysis of field experiments. Am. Nat. 155, 435–453 (2000).
Rosenberg, M., Rothstein, H. & Gurevitch, J. in Handbook of Meta-Analysis in Ecology and Evolution (eds. Koricheva, J. et al.) 61–71 (Princeton Univ. Press, 2013).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010).
Duval, S. & Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463 (2000).
Higgins, J. P. T. & Green, S. in Cochrane Handbook for Systematic Reviews of Interventions (eds. Higgins, J. P. T. & Green, S.) Ch. 9 (Wiley, 2005).
Senior, A. M. et al. Heterogeneity in ecological and evolutionary meta-analyses: its magnitude and implications. Ecology 97, 3293–3299 (2016).
Peck, L. S., Clark, M. S., Morley, S. A., Massey, A. & Rossetti, H. Animal temperature limits and ecological relevance: effects of size, activity and rates of change. Funct. Ecol. 23, 248–256 (2009).
Calcagno, V. glmulti: Model selection and multimodel inference made easy. R package version 1.0.7 (2013).
Kocsis, Á. T., Reddin, C. J., Alroy, J. & Kiessling, W. The R package divDyn for quantifying diversity dynamics using fossil sampling data. Methods Ecol. Evol. 45, 70–84 (2019).
Alroy, J. A more precise speciation and extinction rate estimator. Paleobiology 41, 633–639 (2015).
Clapham, M. E. & Payne, J. L. Acidification, anoxia, and extinction: a multiple logistic regression analysis of extinction selectivity during the Middle and Late Permian. Geology 39, 1059–1062 (2011).
McKinney, M. L. & Oyen, C. W. Causation and nonrandomness in biological and geological time series: temperature as a proximal control of extinction and diversity. Palaios 4, 3–15 (1989).
Emad, A. & Bailey, P. wCorr: Weighted correlations. R package version 1.9.1 (2017).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (grant nos. KI 806/16-1, KO 5382/1-1 and KO 5382/1-2 and Po 278/15-1 and Po 278/16-1) and is embedded in the Research Unit TERSANE (grant no. FOR 2332: Temperature-related stressors as a unifying principle in ancient extinctions). This is Paleobiology Database publication 356 and we thank all database contributors and administrators. H.P. is a co-coordinator for the German BMBF-funded project on BIOACID–Biological Impacts of Ocean Acidification.
Author information
Authors and Affiliations
Contributions
All authors commented on the paper and contributed to revisions. W.K. and H.P. conceived and, with C.J.R., designed the work. C.J.R. and P.N. acquired and analysed the data. C.J.R., W.K., A.K. and H.P. contributed to the interpretation of analyses. C.J.R. and W.K. drafted the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Piero Calosi, Jonathan Payne and Erik Sperling for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Similarities of relative response variation among trait groups between modern experimental CRS responses and fossil mean extinction odds.
Other trait types are shown in Fig. 2. Coefficient error bars denote 95% confidence intervals. Fossil effect sizes are always positive because no grouping made a fossil genus more likely to go extinct than not (that is a negative log-odds) on average over the post-Cambrian Phanerozoic. Modern trait-group estimates are only shown if represented by ≥ 10 species. Fossil models include clade grouping as a random effect.
Extended Data Fig. 2 Response severities (partial effect size, mean and 95% CIs) averaged over all CRS treatments by clades (A), life history stage (B) and response type (C), by clades to single stressors (D-F), and to stressor combinations (G-I).
Horizontal coloured lines show model intercepts. Groups along the x-axis are ordered by rank severity. Other taxa were included in the models but are not displayed because these did not reach sufficient sample sizes for reliable inference (species n ≥ 4). Actual numbers of species coloured above the x-axis. In (a–c), stressor treatment was also included as an undisplayed covariate.
Extended Data Fig. 3 Coverage of (A) living organism source populations involved in CRS response experiments (or, where coordinates were lacking, species distribution centroids), and natural latitudinal trends in (B) temperature and (C) oxygen concentrations.
Experimental start (control) conditions are shown as black filled points linked to end or treatment conditions (red open points) with lines. Dashed lines show LOESS trends. Duplicates removed for correlation tests. *** P < 0.0001. Cold tropical temperatures represent deep-water habitat for diel migratory species. Control salinity ~ absolute latitude, Rho = −0.34, P = 0.007. Ocean pH was unrelated to latitude.
Extended Data Fig. 4 Relationship between taxon fossilisation potential (FP) and acidification response severities.
FP is estimated by the completeness of the fossil record (from earliest sampling) of the modern species’ order (blue) or class (black). Other CRS and genus and family levels were insignificant. Thin lines show slope 95% CIs. Class model, I2 = 77.8%, H2 = 4.5, R2 = 0.009, experiments n = 164. Order model, I2 = 77.5%, H2 = 4.4, R2 = 0.081, experiments n = 136. Cochran’s Q was significant in both cases, P < 0.0001.
Extended Data Fig. 5 The deterioration of temporal match (Rho, logged y-axis) between genus extinction rates of all marine genera and extinction rates of only those genera represented by the classes (blue), orders (green) and families (orange) in our modern marine experimental database.
The time series of Rho values is shown from the median duration of classes, orders or families represented by modern experiments (number in myr). At that (earliest) point in time, the modern experiment-represented families, orders and classes make up 11.3%, 21.8% and 63.1% of total marine genera, respectively. The two extinction rate times series correlated are iteratively expanded back in time by one geological age from the Recent, Pleistocene 1 and 2, that is from the minimum sample size of 3 time bins for a correlation.
Extended Data Fig. 6 Comparing the modern CRS sensitivities with fossil extinction selectivities of selected crisis intervals with CRS hypothesised as drivers.
All details as in Fig. 1. Pseudo-R2 value refers to the binomial regression for the interval of genus extinction (1 or 0) based on clade membership. Correlation 95 % confidence intervals for hypoxia modern-fossil responses is not shown since too few data points (clades) are available. Clade names are below and to the right of points, except ‘Gastropoda’, which is directly above to avoid overlap.
Extended Data Fig. 7 By-clade rank of extinction selectivity compared between mass and background extinction intervals.
Rank is out of 56 clades by extinction log-odds means weighted by extinction rate or its inverse, respectively. Clades with a higher rank selectivity during high extinction rate intervals fall below the x = y diagonal. Larger font highlights the names of clades from Fig. 1a, while clade names we were not able to consider are small.
Extended Data Fig. 8 When does the modern-fossil agreement from Fig. 1 hold or break on average, with ages grouped by extinction intensity quartiles, or by hyperthermal state vs not (based on Harnik et al1.).
The large filled points and thick error bars are the mean correlation ± 95 CIs for the ages in that category (small grey open points). This method, specifically designed for summarizing correlations, required correlations to have clade n > 4, which excludes the end-Permian extinction from the ‘hyperthermal’ category. Mean estimates from another method that permits these ages but is not designed for summarizing correlations (that is, coefficients are not limited between −1 and 1) is provided for comparison (large open points, thin error bars). Methods and category details in Supplementary Methods, ‘Summarizing agreement correlations’.
Extended Data Fig. 9 The quantities of independent experiments by (A) stressor combination, (B) response type, (C) developmental stage and (D) number of species by clade.
Multiple stressor studies, which are prioritized over single-stressor studies in our meta-analysis, often include more treatment levels of combined stressors than of single stressors, hence the large number of combined warming and acidification experiments. ‘Sub-adult’ stages were recoded to ‘adult’. Horizontal red lines show threshold for inference of 20 independent studies in (a) and five species in (d).
Extended Data Fig. 10 Funnel plots of independent responses to test for asymmetry of reporting, showing the difference between if multiple responses per experiment are prioritized (A) by most negative response or (B) by least-variance response.
Residuals are from the basic meta-analytical model with CRS treatment as covariate. (a) suggests a publication bias, that is greater likelihood of reporting high variance responses if they show a strong negative effect size (correlation between effect size and inverse variance, Rho = 0.25, P < 0.0001). Histogram in (c) shows this correlation in context of effect size-error correlations from 100 runs of randomly selecting among multiple responses, with vertical red lines, respectively from left to right showing the correlation in (b, Rho = −0.04, P = 0.23), the median correlation from random runs, and the correlation from (a). Shading in (a) and (B) shows pseudo-confidence interval regions, white = 90%, light grey = 95%, dark grey = 99%.
Supplementary information
Supplementary Information
Supplementary methods, results and Tables 1–7.
Supplementary Table
ROSES for Systematic Review Protocols.
Rights and permissions
About this article
Cite this article
Reddin, C.J., Nätscher, P.S., Kocsis, Á.T. et al. Marine clade sensitivities to climate change conform across timescales. Nat. Clim. Chang. 10, 249–253 (2020). https://doi.org/10.1038/s41558-020-0690-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-0690-7
This article is cited by
-
Diversity, distribution and intrinsic extinction vulnerability of exploited marine bivalves
Nature Communications (2023)
-
Historical warming consistently decreased size, dispersal and speciation rate of fish
Nature Climate Change (2021)
-
Extinction risk controlled by interaction of long-term and short-term climate change
Nature Ecology & Evolution (2021)
-
Thresholds of temperature change for mass extinctions
Nature Communications (2021)
-
Morphological response accompanying size reduction of belemnites during an Early Jurassic hyperthermal event modulated by life history
Scientific Reports (2021)