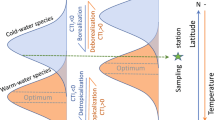
Abstract
Poleward range extensions by warm-adapted sea urchins are switching temperate marine ecosystems from kelp-dominated to barren-dominated systems that favour the establishment of range-extending tropical fishes. Yet, such tropicalization may be buffered by ocean acidification, which reduces urchin grazing performance and the urchin barrens that tropical range-extending fishes prefer. Using ecosystems experiencing natural warming and acidification, we show that ocean acidification could buffer warming-facilitated tropicalization by reducing urchin populations (by 87%) and inhibiting the formation of barrens. This buffering effect of CO2 enrichment was observed at natural CO2 vents that are associated with a shift from a barren-dominated to a turf-dominated state, which we found is less favourable to tropical fishes. Together, these observations suggest that ocean acidification may buffer the tropicalization effect of ocean warming against urchin barren formation via multiple processes (fewer urchins and barrens) and consequently slow the increasing rate of tropicalization of temperate fish communities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (2006).
Pecl, G. T. et al. Biodiversity redistribution under climate: impacts on ecosystems and human well-being. Science 355, eaai9214 (2017).
Ling, S. D. Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156, 883–894 (2008).
Feary, D. A. et al. Latitudinal shift in coral reef fishes: why some species do other do not shift. Fish. Fish. (Oxf.) 15, 593–615 (2013).
Nakamura, Y., Feary, D. A., Kanda, M. & Yamaoka, K. Tropical fishes dominate temperate reef fish communities within western Japan. PLoS ONE 8, e81107 (2013).
Peers, M. J. L., Wehtje, M., Thornton, D. H. & Murray, D. L. Prey switching as a means of enhancing persistence in predators at the trailing southern edge. Glob. Change Biol. 20, 1126–1135 (2014).
Verges, A. et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl Acad. Sci. USA 113, 13791–13796 (2016).
Ling, S. D., Johnson, C. R., Ridgway, K., Hobday, A. J. & Haddo, M. Climate-driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics. Glob. Change Biol. 15, 719–731 (2009).
Johnson, C. R., Ling, S. D., Ross, J., Shepherd, S. & Miller, K. Establishment of the Long-Spined Sea Urchin (Centrostephanus rodgersii) in Tasmania: First Assessment of Potential Threats to Fisheries. FRDC Final Report, Project No. 2001/044 (School of Zoology & Tasmanian Aquaculture and Fisheries Institute, University of Tasmania, 2005).
Beck, H. J., Feary, D. A., Nakamura, Y. & Booth, D. J. Temperate macroalgae impacts tropical fish recruitment at forefront of range expansion. Coral Reefs 36, 639–651 (2017).
Nagelkerken, I. & Connell, S. D. Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions. Proc. Natl Acad. Sci. USA 112, 13272–13277 (2015).
Wernberg, T. et al. Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172 (2016).
Connell, S. D. et al. The duality of ocean acidification as a resource and a stressor. Ecology 99, 1005–1010 (2018).
Nagelkerken, I., Goldenberg, S. U., Ferreira, C. M., Russell, B. D. & Connell, S. D. Species interactions drive fish biodiversity loss in a high-CO2 world. Curr. Biol. 27, 2177–2184 (2017).
Sunday, J. M. et al. Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat. Clim. Change 7, 81–85 (2017).
Connell, S. D., Kroeker, K. J., Fabricius, K. E., Kline, D. I. & Russell, B. D. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Proc. R. Soc. B 368, 20120442 (2013).
Russell, B. D. et al. Future seagrass beds: can increased productivity lead to increased carbon storage? Mar. Pollut. Bull. 73, 463–469 (2013).
Palacios, S. L. & Zimmerman, R. C. Response of ellgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Mar. Ecol. Prog. Ser. 344, 1–13 (2007).
Hepburn, C. D. et al. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Glob. Change Biol. 17, 2488–2497 (2011).
Linares, C. et al. Persistent natural acidification drives major distribution shifts in marine benthic ecosystems. Proc. R. Soc. B Biol. Sci. 282, 20150587 (2015).
Russell, B. D., Thompson, J. A. I., Falkenberg, L. J. & Connell, S. D. Synergistic effects of climate change and local stressors: CO2 and nutrient-driven change in subtidal rocky habitats. Glob. Change Biol. 15, 2153–2162 (2009).
Connell, S. D. & Russell, B. D. The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests. Proc. R. Soc. B Biol. Sci. 277, 1409–1415 (2010).
Diaz-Pulido, G., Gouezo, M., Tilbrook, B., Dove, S. & Anthony, K. R. N. High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 14, 156–162 (2011).
Johnson, M. D., Comeau, S., Lantz, C. A. & Smith, J. E. Complex and interactive effects of ocean acidification and temperature on epilithic and endolithic coral-reef turf algal assemblages. Coral Reefs 36, 1059–1070 (2017).
Kroeker, K. J., Kordas, R. L. & Harley, D. G. Embracing interactions in ocean acidification research: confronting multiple stressor scenarios and context dependence. Biol. Lett. 13, 20160802 (2017).
Goldenberg, S. U., Nagelkerken, I., Ferreira, C. M., Ullah, H. & Connell, S. D. Boosted food web productivity through ocean acidification collapses under warming. Glob. Change Biol. 23, 4177–4184 (2017).
Wernberg, T., Smale, D. A. & Thomsen, M. S. A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob. Change Biol. 18, 1491–1498 (2012).
Kroeker, K. J., Micheli, F., Gambi, M. C. & Martz, T. R. Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl Acad. Sci. USA 108, 14515–14520 (2011).
Goldenberg, S. U. et al. Ecological complexity buffers the impacts of future climate on marine consumers. Nat. Clim. Change 8, 229–233 (2018).
Connell, S. D. & Ghedini, G. Resisting regime-shifts: the stabilising effect of compensatory processes. Trends Ecol. Evol. 30, 513–515 (2015).
Widdicombe, S., Dupont, S. & Thorndyke, M. Laboratory Experiments and Benthic Mesocosm Studies. Guide for Best Practices in Ocean Acidification Research and Data Reporting (EPOCA, 2008).
Hofmann, G. E. et al. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 (2011).
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003).
Hoegh-Guldberg, O. & Bruno, J. F. The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528 (2010).
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245 (2013).
Ling, S. D. et al. Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil. Trans. R. Soc. B 370, 20130269 (2015).
Calosi, P. et al. Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid–base and ion-regulatory abilities. Mar. Pollut. Bull. 73, 470–484 (2013).
Booth, D. J., Figueira, W. F., Gregson, M. A., Brown, L. & Beretta, G. Occurrence of tropical fishes in temperate southeastern Australia: role of the East Australian Current. Estuar. Coast. Shelf Sci. 72, 102–114 (2007).
Nagelkerken, I., Russell, B. D., Gillanders, B. M. & Connell, S. D. Ocean acidification alters fish populations indirectly through habitat modification. Nat. Clim. Change 6, 89–93 (2016).
Hall-Spencer, J. et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 (2008).
Kroeker, K., Gambi, M. C. & Micheli, F. Community dynamics and ecosystem simplification in a high-CO2 ocean. Proc. Natl Acad. Sci. USA 110, 12721–12726 (2013).
Enochs, I. C. et al. Shift from coral to macroalgae dominance on volcanically acidified reef. Nat. Clim. Change 5, 1083–1088 (2015).
Suding, K. N. & Hobbs, R. J. Threshold models in restoration and conservation: a developing framework. Trends Ecol. Evol. 24, 271–279 (2009).
Perry, A. L., Low, O. L., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (2005).
Steneck, R. S. Herbivory on coral reefs: a synthesis. In Proc. 6th International Coral Reef Symposium. Vol. 1, 37–49 (1988).
Purcell, S. W. & Bellwood, D. R. A functional analysis of food procurement in two surgeonfish species, Acanthurus nigrofuscus and Ctenochaetus striatus (Acanthuridae). Environ. Biol. Fishes 37, 139–159 (1993).
Curley, B. G., Gillanders, B. M. & Kingsford, M. J. Spatial and habitat related patterns of temperate reef fish assemblages: implications for the design of marine protected areas. Mar. Freshw. Res. 53, 1197–1210 (2002).
Coen, L. D., Luckenbach, M. W. & Breitburg, D. L. The role of oyster reef as essential fish habitat: a review of current knowledge and some new perspectives. Am. Fish. Soc. Symp. 22, 438–454 (1999).
Lenihan, H. S. et al. Cascading of habitat degradation: oyster reefs invaded by refugee fishes escaping stress. Ecol. Appl. 11, 764–782 (2001).
Jackson, J. B. C. et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637 (2001).
Thomas, Y., Cassou, C., Gernez, P. & Pouvreau, S. Oysters as sentinels of climatic variability and climatic change in coastal ecosystems. Environ. Res. Lett. 13, 104009 (2018).
Alleway, H. K. & Connell, S. D. Loss of an ecological baseline through the eradication of oyster reefs from coastal ecosystems and human memory. Conserv. Biol. 29, 795–804 (2015).
Filbee-Dexter, K. & Wernberg, T. Rise of turfs: a new battlefront for globally declining kelp forests. BioScience 168, 64–76 (2018).
O’Brien, J. M. & Scheibling, R. E. Turf wars: competition between foundation and turf-forming species on temperate and tropical reefs and its role in regime shifts. Mar. Ecol. Prog. Ser. 599, 1–17 (2018).
Vergés, A. et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B Biol. Sci. 281, 20140846 (2014).
Bulleri, F., Bruno, J. F., Silliman, B. R. & Stachowicz, J. J. Facilitation and the niche: implications for coexistence, range shifts and ecosystem functioning. Funct. Ecol. 30, 70–78 (2016).
Smith, S. M., Fox, R. J., Booth, D. J. & Donelson, J. M. ‘Stick with your kind, or hang with locals?’ Implications of shoaling strategy for tropical reef fish on a range-expansion frontline. Glob. Change Biol. 24, 1663–1672 (2018).
Kingsbury, K. M., Gillanders, B. M., Booth, D. J., Coni, E. O. C. & Nagelkerken, I. Range-extending coral reef fishes trade-off growth for maintenance of body condition in cooler waters. Sci. Total Environ. 703, 134598 (2019).
Kingsbury, K. M., Gillanders, B. M., Booth, D. J. & Nagelkerken, I. Trophic niche segregation allows range-extending coral reef fishes to co-exist with temperate species under climate change. Glob. Change Biol. 26, 721–733 (2020).
Foo, S. A., Dworjanyn, S. A., Poore, A. G. B. & Byrne, M. Adaptive capacity of the habitat modifying sea urchin Centrostephanus rodgersii to ocean warming and ocean acidification: performance of early embryos. PLoS ONE 7, e42497 (2012).
Kelly, M. W., Padilla-Gamino, J. & Hofmann, G. E. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrus purpuratus. Glob. Change Biol. 19, 2536–2546 (2013).
Uthicke, S. et al. Little evidence of adaptation potential to ocean acidification at a CO2 vent. Ecol. Evol. 9, 10004–10016 (2019).
Somero, G. N. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 (2010).
Siikayuopio, A. I., Mortesen, A., Dale, T. & Foss, A. Effects of carbon dioxide exposure on feed intake and gonad growth in green sea urchin, Stringylicentritus droebachiensis. Aquaculture 266, 97–101 (2007).
Dworjanyn, S. A. & Byrne, M. Impacts of ocean acidification on sea urchin growth across the juvenile to mature adult life-stage transition is mitigated by warming. Proc. R. Soc. B Biol. Sci. 285, 20172684 (2018).
Miles, H., Widdicombe, S., Spicer, J. I. & Hall-Spencer, J. Effects of anthropogenic seawater acidification on acid–base balance in the sea urchin Psammechinus miliaris. Mar. Pollut. Bull. 54, 89–96 (2007).
Spicer, J. I., Widdicombe, S., Needham, H. R. & Berge, J. A. Impact of CO2-acidified seawater on the extracellular acid–base balance of the northern sea urchin Strongylocentrotus dröebachiensis. J. Exp. Mar. Biol. Ecol. 407, 19–25 (2011).
Uthicke, S. et al. Echinometra sea urchins acclimatized to elevated pCO2 at volcanic vents outperform those under present-day pCO2 conditions. Glob. Change Biol. 22, 2451–2461 (2016).
Wernberg, T. et al. Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecol. Lett. 13, 685–694 (2010).
Simonson, E. J., Metaxas, A. & Scheibling, R. E. Kelp in hot water: effects of warming seawater temperature on kelp quality as a food source and settlement substrate. Mar. Ecol. Prog. Ser. 537, 105–119 (2015).
Ross, P. M., Parker, L. & Byrne, M. Transgenerational responses of molluscs and echinoderms to changing ocean conditions. ICES J. Mar. Sci. 73, 537–549 (2016).
Wong, J. M., Johnson, K. M., Kelly, M. W. & Hofmann, G. E. Transcriptomics reveals transgenerational effects in purple sea urchin embryos: adult acclimation to upwelling conditions alters the response of their progeny to differential pCO2 levels. Mol. Ecol. 27, 1120–1137 (2018).
Clark, M. S. et al. Molecular mechanisms underpinning transgenerational plasticity in the green sea urchin Psammechinus miliaris. Sci. Rep. 9, 952 (2019).
Ghedini, G., Russell, B. D. & Connell, S. D. Trophic compensation reinforces resistance: herbivory absorbs the increasing effects of multiple disturbances. Ecol. Lett. 18, 182–187 (2015).
Munday, P. L., Rummer, J. L. & Baumann, H. Adaptation and evolutionary responses to high CO2. Fish. Physiol. 37, 369–395 (2019).
Miller, G. M., Watson, S. A., Donelson, J. M., McCormick, M. I. & Munday, P. L. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2, 858–861 (2012).
Allan, B. J. M., Miller, G. M., McCormick, M. I., Domenici, P. & Munday, P. L. Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc. R. Soc. B Biol. Sci. 281, 20132179 (2014).
Welch, M., Watson, S., Welsh, J. Q., McCormick, M. I. & Munday, P. L. Effect of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat. Clim. Change 4, 1086–1089 (2014).
Rummer, J. L. & Munday, P. L. Climate change and the evolution of reef fishes: past and future. Fish. Fish. (Oxf.) 18, 22–39 (2017).
Connell, S. D. & Irving, A. D. Integrating ecology with biogeography using landscape characteristics: a case study of subtidal habitat across continental Australia. J. Biogeogr. 35, 1608–1621 (2008).
Pecorino, D., Lamare, M. D. & Barker, M. F. Growth, morphometrics and size structure of the Diamatidae sea urchin Centrostephanus rodgersii in northern New Zealand. Mar. Freshw. Res. 63, 624–634 (2012).
Brinkman, T. J. & Smith, A. M. E. Effects of climate change on crustose coralline algae at a temperate vent site, White Island, New Zealand. Mar. Freshw. Res. 66, 360–370 (2015).
Hughes, T. P. et al. Coral reefs in the Anthropocene. Nature 596, 82–90 (2017).
Booth, D. J., Beretta, G. A., Brown, L. & Figueira, W. F. Predicting success of range-expanding coral reef fish in temperate habitats using fish in temperature–abundance relationships. Front. Mar. Sci. 5, 31 (2018).
Ridgeway, K. R. Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophys. Res. Lett. 34, L13613 (2007).
Hobday, A. J. & Pecl, G. T. Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Rev. Fish Biol. Fish. 24, 415–425 (2013).
Figueira, W. F. & Booth, D. J. Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters. Glob. Change Biol. 16, 506–516 (2010).
McLeod, I. et al. Habitat value of Sydney rock oyster (Saccostrea glomerata) reefs on soft sediments. Mar. Freshw. Res. 71, 771–781 (2019).
Gillies, C. L. et al. Australian shellfish ecosystems: past distribution, current status and future direction. PLoS ONE 13, e0190914 (2018).
Minte-Vera, C. V., Moura, R. L. & Francini-Filho, R. B. Nested sampling: an improved visual-census technique for studying reef fish assemblages. Mar. Ecol. Prog. Ser. 367, 283–293 (2008).
Fulton, C. J., Noble, M. N., Radford, B., Gallen, C. & Harasti, D. Microhabitat selectivity underpins regional indicators of fish abundance and replenishment. Ecol. Indic. 70, 222–231 (2016).
Choat, J. H. & Clements, K. D. Diet in Odacid and Aplodactylid fishes from Australia and New Zealand. Aust. J. Mar. Freshw. Res. 43, 1451–1459 (1992).
Clements, K. D. & Choat, J. H. Comparison of herbivory in the closely-related marine fish genera Girella and Kyphosus. Mar. Biol. 127, 579–586 (1997).
Ceccarelli, D. M. Modification of benthic communities by territorial damselfish: a multi-species comparison. Coral Reefs 26, 853–866 (2007).
Zarco-Perello, S., Wemberg, T., Langlois, T. J. & Vanderklift, M. A. Tropicalization strengthens consumer pressure on habitat-forming seaweeds. Sci. Rep. 7, 820 (2017).
Anderson, M. J. & Willis, T. J. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84, 511–525 (2003).
Paliy, O. & Shankar, V. Application of multivariate statistical techniques in microbial ecology. Mol. Ecol. 25, 1032–1057 (2016).
Hemingson, C. R. & Bellwood, D. R. Biogeographic patterns in major marine realms: function not taxonomy unites fish assemblages in reef, seagrass and mangrove systems. Ecography 41, 174–182 (2018).
McClanahan, T. R. & Kaunda-Arara, B. Fishery recovery in a coral-reef marine park and its effect on the adjacent fishery. Conserv. Biol. 10, 1187–1199 (1996).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26, 32–46 (2001).
Wernberg, T. et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Change 3, 78–82 (2013).
Johnson, C. R. et al. Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J. Exp. Mar. Biol. Ecol. 400, 17–32 (2011).
Scheffer, M. Critical Transitions in Nature and Society (Princeton Univ. Press, 2009).
Jax, K. Thresholds, tipping points and limits. In OpenNESS Ecosystem Services Reference Book (eds Potschin, M. & Jax, K.) (2016).
Acknowledgements
We thank K. Kingsbury, M. Sasaki and M. Krutz for logistic support in the field. This project was funded by Australian Research Council (ARC) Discovery Project DP170101722 to I.N. and D.J.B. Additional financial support was provided by an ARC Future Fellowship to I.N. (grant number FT120100183), an ARC Discovery Project to S.D.C. (grant number DP150104263) and a grant from the Environment Institute (University of Adelaide).
Author information
Authors and Affiliations
Contributions
E.O.C.C., I.N., D.J.B. and S.D.C. conceived of and designed the study. E.O.C.C. and C.M.F. collected the data. E.O.C.C. analysed the data. E.O.C.C., I.N., D.J.B. and S.D.C. wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Cristina Linares and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Conceptual models of hysteresis under different climate change stressors.
Regime shifts from kelp forests (green solid lines) to alternative turf and barren-dominated states (orange and pink solid lines) and the occurrence of hysteresis under different climate change scenarios: (a) ocean warming12,101, (b) ocean acidification16, (c) urchin overgrazing3,102, and (d) all three stressors combined (present study). When the stressors are strong enough, and ecosystem state 1 passes beyond the tipping point (T1), a discontinuous critical transition occurs from an unstable equilibrium (dashed line) to the alternative stable state 2 (degradation) (downward black arrows). However, if stressor levels are then reduced, a hysteresis occurs because the opposing forces fail to push the ecosystem to return to its original state. The recovery to state 1 is only possible if the magnitude of the stressors is reduced to a much lower level (T2) (upward black arrows) than that of the tipping point during the degradation. Adapted from Scheffer103 and Jax104.
Extended Data Fig. 2 Tropicalisation hotspot and CO2 vent study areas.
Map showing the three tropicalisation hotspots in Sydney (Australia) where tropical and temperate fish communities were surveyed, and the CO2 vents at White Island (New Zealand) where the effects of elevated CO2 on fish communities and sea-urchins were investigated.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Tables 1–7.
Rights and permissions
About this article
Cite this article
Coni, E.O.C., Nagelkerken, I., Ferreira, C.M. et al. Ocean acidification may slow the pace of tropicalization of temperate fish communities. Nat. Clim. Chang. 11, 249–256 (2021). https://doi.org/10.1038/s41558-020-00980-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-00980-w
This article is cited by
-
Impacts of ocean acidification on physiology and ecology of marine invertebrates: a comprehensive review
Aquatic Ecology (2023)
-
Projected ocean acidification and seasonal temperature alter the behaviour and growth of a range extending tropical fish
Coral Reefs (2023)
-
Novel species interactions and environmental conditions reduce foraging competency at the temperate range edge of a range-extending coral reef fish
Coral Reefs (2021)