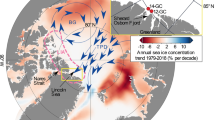
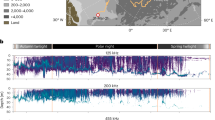
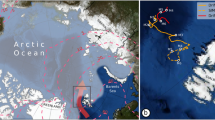
Abstract
The Arctic sea-ice-scape is rapidly transforming. Increasing light penetration will initiate earlier seasonal primary production. This earlier growing season may be accompanied by an increase in ice algae and phytoplankton biomass, augmenting the emission of dimethylsulfide and capture of carbon dioxide. Secondary production may also increase on the shelves, although the loss of sea ice exacerbates the demise of sea-ice fauna, endemic fish and megafauna. Sea-ice loss may also deliver more methane to the atmosphere, but warmer ice may release fewer halogens, resulting in fewer ozone depletion events. The net changes in carbon drawdown are still highly uncertain. Despite large uncertainties in these assessments, we expect disruptive changes that warrant intensified long-term observations and modelling efforts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arrigo, K. R. in Sea Ice (Ed. Thomas, D. N.) 352–369 (John Wiley & Sons, Ltd, 2017).
Steiner, N. S. et al. Impacts of the changing ocean-sea ice system on the key forage fish Arctic cod (Boreogadus saida) and subsistence fisheries in the western Canadian Arctic—evaluating linked climate, ecosystem and economic (CEE) models. Front. Mar. Sci. 6, 179 (2019).
Kohlbach, D. et al. The importance of ice algae-produced carbon in the central Arctic Ocean ecosystem: food web relationships revealed by lipid and stable isotope analyses. Limnol. Oceanogr. 61, 2027–2044 (2016).
Boetius, A. et al. Export of algal biomass from the melting Arctic sea ice. Science 339, 1430–1432 (2013).
Riebesell, U., Schloss, I. & Smetacek, V. Aggregation of algae released from melting sea ice: implications for seeding and sedimentation. Polar Biol. 11, 239–248 (1991).
MacGilchrist, G. A. et al. The Arctic Ocean carbon sink. Deep. Res. Part I Oceanogr. Res. Pap. 86, 39–55 (2014).
Bates, N. R. & Mathis, J. T. The Arctic Ocean marine carbon cycle: evaluation of air-sea CO2 exchanges, ocean acidification impacts and potential feedbacks. Biogeosciences 6, 2433–2459 (2009).
Notz, D. & Stroeve, J. Observed Arctic sea-ice loss directly follows anthropogenic CO2 emission. Science 354, 747–750 (2016).
Meier, W. N. et al. Arctic sea ice in transformation: a review of recent observed changes and impacts on biology and human activity. Rev. Geophys. 52, 185–217 (2014).
Kwok, R. Arctic sea ice thickness, volume, and multiyear ice coverage: losses and coupled variability (1958–2018). Environ. Res. Lett. 13, 105005 (2018).
Maslanik, J., Stroeve, J., Fowler, C. & Emery, W. Distribution and trends in Arctic sea ice age through spring 2011. Geophys. Res. Lett. 38, L13502 (2011).
Stroeve, J. C., Crawford, A. D. & Stammerjohn, S. Using timing of ice retreat to predict timing of fall freeze-up in the Arctic. Geophys. Res. Lett. 43, 6332–6340 (2016).
Webster, M. A. et al. Interdecadal changes in snow depth on Arctic sea ice. J. Geophys. Res. Ocean. 119, 5395–5406 (2014).
Strong, C. & Rigor, I. G. Arctic marginal ice zone trending wider in summer and narrower in winter. Geophys. Res. Lett. 40, 4864–4868 (2013).
IPCC Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) 1029–1136 (Cambridge Univ. Press, 2013).
Overland, J. E. & Wang, M. When will the summer Arctic be nearly sea ice free? Geophys. Res. Lett. 40, 2097–2101 (2013).
Bintanja, R. & Andry, O. Towards a rain-dominated Arctic. Nat. Clim. Change 7, 263–267 (2017).
Vancoppenolle, M. et al. Role of sea ice in global biogeochemical cycles: emerging views and challenges. Quat. Sci. Rev. 79, 207–230 (2013).
Berge, J. et al. In the dark: a review of ecosystem processes during the Arctic polar night. Prog. Oceanogr. 139, 258–271 (2015).
Leu, E. et al. Arctic spring awakening — steering principles behind the phenology of vernal ice algal blooms. Prog. Oceanogr. 139, 151–170 (2015).
Assmy, P. et al. Leads in Arctic pack ice enable early phytoplankton blooms below snow-covered sea ice. Sci. Rep. 7, 40850 (2017).
Perovich, D. K. Sea Ice (Ed. Thomas, D. N.) 110–137 (John Wiley & Sons, Ltd, 2017).
Nicolaus, M., Katlein, C., Maslanik, J. A. & Hendricks, S. Solar Radiation Over and Under Sea Ice During the POLARSTERN Cruise ARK-XXVI/3 (TransArc) in Summer 2011 (PANGAEA, 2011); https://doi.pangaea.de/10.1594/PANGAEA.786717
Arrigo, K. R. et al. Massive phytoplankton blooms under Arctic sea ice. Science 336, 1408 (2012).
Pistone, K., Eisenman, I. & Ramanathan, V. Observational determination of albedo decrease caused by vanishing Arctic sea ice. Proc. Natl Acad. Sci. USA 111, 3322–3326 (2014).
Horvat, C. et al. The frequency and extent of sub-ice phytoplankton blooms in the Arctic Ocean. Sci. Adv. 3, e1601191 (2017).
El-Sayed, S. Z., Van Dijken, G. L. & Gonzalez-Rodas, G. Effects of ultraviolet radiation on marine ecosystems. Int. J. Environ. Stud. 51, 199–216 (1996).
Elliott, A. et al. Spring production of mycosporine-like amino acids and other UV-absorbing compounds in sea ice-associated algae communities in the Canadian Arctic. Mar. Ecol. Prog. Ser. 541, 91–104 (2015).
Ryan, K. G., Mcminn, A., Hegseth, E. N. & Davy, S. K. The effects of ultraviolet-b radiation on antarctic sea-ice algae. J. Phycol. 48, 74–84 (2012).
Arrigo, K. R. & van Dijken, G. L. Continued increases in Arctic Ocean primary production. Prog. Oceanogr. 136, 60–70 (2015).
Gradinger, R. Sea-ice algae: major contributors to primary production and algal biomass in the Chukchi and Beaufort Seas during May/June 2002. Deep. Res. Part II Top. Stud. Oceanogr. 56, 1201–1212 (2009).
Tremblay, J.-E. & Gagnon, J. in Influence of Climate Change on the Changing Arctic and Sub-Arctic Conditions (eds Nihoul, J. C. J. & Kostianoy, A. G.) 73–93 (Springer, 2009).
Nomura, D. et al. Nutrient distributions associated with snow and sediment-laden layers in sea ice of the southern Sea of Okhotsk. Mar. Chem. 119, 1–8 (2010).
Meiners, K. M. & Michel, C. in Sea Ice (Ed. Thomas, D. N.) 415–432 (John Wiley & Sons, Ltd, 2017).
Fripiat, F. et al. Macro-nutrient concentrations in Antarctic pack ice: overall patterns and overlooked processes. Elem. Sci. Anth. 5, p13 (2017).
Tremblay, J. É. et al. Global and regional drivers of nutrient supply, primary production and CO2 drawdown in the changing Arctic Ocean. Prog. Oceanogr. 139, 171–196 (2015).
Miller, J. R. & Russell, G. L. Projected impact of climate change on the freshwater and salt budgets of the Arctic Ocean by a global climate model. Geophys. Res. Lett. 27, 1183–1186 (2000).
Peterson, B. J. et al. Increasing river discharge to the Arctic Ocean. Science 298, 2171–2173 (2002).
Rainville, L., M. Lee, C. & Woodgate, A. R. Impact of wind-driven mixing in the Arctic Ocean. Oceanography 24, 136–145 (2011).
Lamarque, J. F. et al. Multi-model mean nitrogen and sulfur deposition from the atmospheric chemistry and climate model intercomparison project (ACCMIP): evaluation of historical and projected future changes. Atmos. Chem. Phys. 13, 7997–8018 (2013).
Stroeve, J. C., Markus, T., Boisvert, L., Miller, J. & Barrett, A. Changes in Arctic melt season and implications for sea ice loss. Geophys. Res. Lett. 41, 1216–1225 (2014).
Tedesco, L., Vichi, M. & Scoccimarro, E. Sea-ice algal phenology in a warmer Arctic. Sci. Adv. 5, eaav4830 (2019).
van Leeuwe, M. A. et al. Microalgal community structure and primary production in Arctic and Antarctic sea ice: a synthesis. Elem. Sci. Anth. https://doi.org/10.1525/elementa.267 (2018).
Hardge, K. et al. Sea ice origin and sea ice retreat as possible drivers of variability in Arctic marine protist composition. Mar. Ecol. Prog. Ser. 571, 43–57 (2017).
Campbell, K., Mundy, C. J., Belzile, C., Delaforge, A. & Rysgaard, S. Seasonal dynamics of algal and bacterial communities in Arctic sea ice under variable snow cover. Polar Biol. 41, 41–58 (2018).
Leu, E., Søreide, J. E., Hessen, D. O., Falk-Petersen, S. & Berge, J. Consequences of changing sea-ice cover for primary and secondary producers in the European Arctic shelf seas: timing, quantity, and quality. Prog. Oceanogr. 90, 18–32 (2011).
Fernández-Méndez, M. et al. Composition, buoyancy regulation and fate of ice algal aggregates in the Central Arctic Ocean. PLoS ONE 9, e107452 (2014).
Ardyna, M. et al. Recent Arctic Ocean sea ice loss triggers novel fall phytoplankton blooms. Geophys. Res. Lett. 41, 6207–6212 (2014).
Wassmann, P. & Reigstad, M. Future Arctic Ocean seasonal ice zones and implications for pelagic-benthic coupling. Oceanography 24, 220–231 (2011).
Dalman, L. et al. Enhanced bottom-ice algal biomass across a tidal strait in the Kitikmeot Sea of the Canadian Arctic. Elem. Sci. Anth. 7, p22 (2019).
Williams, W. et al. Joint effects of wind and ice motion in forcing upwelling in Mackenzie Trough, Beaufort Sea. Cont. Shelf Res. 26, 2352–2366 (2006).
Ardyna, M. et al. Environmental drivers of under-ice phytoplankton bloom dynamics in the Arctic Ocean. Elem. Sci. Anth. 8, 30 (2020).
Eronen-Rasimus, E. et al. Ice formation and growth shape bacterial community structure in Baltic Sea drift ice. FEMS Microbiol. Ecol. 91, 1–13 (2015).
Bowman, J. S. The relationship between sea ice bacterial community structure and biogeochemistry: a synthesis of current knowledge and known unknowns. Elem. Sci. Anthr. 3, 000072 (2015).
Eronen-Rasimus, E. et al. An active bacterial community linked to high chl-a concentrations in Antarctic winter-pack ice and evidence for the development of an anaerobic sea-ice bacterial community. ISME J. 11, 2345–2355 (2017).
Kohlbach, D. et al. The importance of ice algae-produced carbon in the central Arctic Ocean ecosystem: food web relationships revealed by lipid and stable isotope analyses. Limnol. Oceanogr. 61, 2027–2044 (2016).
Fossheim, M. et al. Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat. Clim. Change 5, 673–677 (2015).
Søreide, J. E., Leu, E. V. A., Berge, J., Graeve, M. & Falk-Petersen, S. Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob. Chang. Biol. 16, 3154–3163 (2010).
Eriksen, E., Skjoldal, H. R., Gjøsæter, H. & Primicerio, R. Spatial and temporal changes in the Barents Sea pelagic compartment during the recent warming. Prog. Oceanogr. 151, 206–226 (2017).
David, C., Lange, B., Rabe, B. & Flores, H. Community structure of under-ice fauna in the Eurasian central Arctic Ocean in relation to environmental properties of sea-ice habitats. Mar. Ecol. Prog. Ser. 522, 15–32 (2015).
Melnikov, I. Recent Arctic sea-ice ecosystem: dynamics and forecast. Dokl. Earth Sci. 423, 1516–1519 (2008).
Haug, T. et al. Future harvest of living resources in the Arctic Ocean north of the Nordic and Barents Seas: a review of possibilities and constraints. Fish. Res. 188, 38–57 (2017).
Kędra, M. et al. Status and trends in the structure of Arctic benthic food webs. Polar Res. 34, 23775 (2015).
Filbee-Dexter, K., Wernberg, T., Fredriksen, S., Norderhaug, K. M. & Pedersen, M. F. Arctic kelp forests: diversity, resilience and future. Glob. Planet. Change 172, 1–14 (2019).
Murillo, F. J. et al. Sponge assemblages and predicted archetypes in the eastern Canadian Arctic. Mar. Ecol. Prog. Ser. 597, 115–135 (2018).
Hamilton, C. D., Lydersen, C., Ims, R. A. & Kovacs, K. M. Predictions replaced by facts: a keystone species’ behavioural responses to declining arctic sea-ice. Biol. Lett. 11, 20150803 (2015).
O’Corry-Crowe, G. et al. Genetic profiling links changing sea-ice to shifting beluga whale migration patterns. Biol. Lett. 12, 20160404 (2016).
Descamps, S. et al. Climate change impacts on wildlife in a High Arctic archipelago — Svalbard, Norway. Glob. Chang. Biol. 23, 490–502 (2017).
Wollenburg, J. E. et al. Ballasting by cryogenic gypsum enhances carbon export in a Phaeocystis under-ice bloom. Sci. Rep. 8, 7703 (2018).
Darnis, G. & Fortier, L. Zooplankton respiration and the export of carbon at depth in the Amundsen Gulf (Arctic Ocean). J. Geophys. Res. 117, C04013 (2012).
Darnis, G. et al. From polar night to midnight sun: diel vertical migration, metabolism and biogeochemical role of zooplankton in a high Arctic fjord (Kongsfjorden, Svalbard). Limnol. Oceanogr. 62, 1586–1605 (2017).
Wiedmann, I., Reigstad, M., Sundfjord, A. & Basedow, S. Potential drivers of sinking particle’s size spectra and vertical flux of particulate organic carbon (POC): turbulence, phytoplankton, and zooplankton. J. Geophys. Res. Ocean. 119, 6900–6917 (2014).
Flores, H. et al. Sea-ice properties and nutrient concentration as drivers of the taxonomic and trophic structure of high-Arctic protist and metazoan communities. Polar Biol. 42, 1377–1395 (2019).
Belcher, A. et al. The potential role of Antarctic krill faecal pellets in efficient carbon export at the marginal ice zone of the South Orkney Islands in spring. Polar Biol. 40, 2001–2013 (2017).
Lalande, C. et al. Variability in under-ice export fluxes of biogenic matter in the Arctic Ocean. Global Biogeochem. Cycles 28, 571–583 (2014).
Miller, L. A., Carnat, G., Else, B. G. T., Sutherland, N. & Papakyriakou, T. N. Carbonate system evolution at the Arctic Ocean surface during autumn freeze-up. J. Geophys. Res. Ocean. 116, C00G04 (2011).
Dieckmann, G. S. et al. Brief Communication: ikaite (CaCO3·6H2O) discovered in Arctic sea ice. Cryosphere 4, 227–230 (2010).
Rysgaard, S. et al. Ikaite crystals in melting sea ice — implications for pCO2 and pH levels in Arctic surface waters. Cryosphere 6, 901–908 (2012).
Nomura, D. et al. CO2 flux over young and snow-covered Arctic pack ice in winter and spring. Biogeosciences 15, 3331–3343 (2018).
König, D., Miller, L. A., Simpson, K. G. & Vagle, S. Carbon dynamics during the formation of sea ice at different growth rates. Front. Earth Sci. 6, 234 (2018).
Grimm, R., Notz, D., Glud, R. N., Rysgaard, S. & Six, K. D. Assessment of the sea-ice carbon pump: insights from a three-dimensional ocean-sea-ice-biogeochemical model (MPIOM/HAMOCC). Elem. Sci. Anthr. 4, 000136 (2016).
Rysgaard, S., Glud, R. N., Sejr, M. K., Bendtsen, J. & Christensen, P. B. Inorganic carbon transport during sea ice growth and decay: a carbon pump in polar seas. J. Geophys. Res. 112, C03016 (2007).
Manizza, M. et al. Changes in the Arctic Ocean CO2 sink (1996–2007): a regional model analysis. Global Biogeochem. Cycles 27, 1108–1118 (2013).
Mortenson, E. Modelling carbon exchange in the air, sea, and ice of the Arctic Ocean. PhD thesis, Univ. of Victoria (2019).
Fransson, A. et al. Effects of sea-ice and biogeochemical processes and storms on under-ice water fCO2 during the winter-spring transition in the high Arctic Ocean: implications for sea-air CO2 fluxes. J. Geophys. Res. Ocean. 122, 5566–5587 (2017).
Mathis, J. T. et al. Storm-induced upwelling of high pCO2 waters onto the continental shelf of the western Arctic Ocean and implications for carbonate mineral saturation states. Geophys. Res. Lett. 39, L07606 (2012).
Pipko, I. I., Semiletov, I. P., Pugach, S. P., Wählstrãm, I. & Anderson, L. G. Interannual variability of air-sea CO2 fluxes and carbon system in the East Siberian Sea. Biogeosciences 8, 1987–2007 (2011).
Steiner, N. et al. What sea-ice biogeochemical modellers need from observers. Elementa 4, 000084 (2016).
Cai, W.-J. et al. Decrease in the CO2 uptake capacity in an ice-free Arctic Ocean Basin. Science 329, 556–559 (2010).
Else, B. et al. Further observations of a decreasing atmospheric CO2 uptake capacity in the Canada Basin (Arctic Ocean) due to sea ice loss. Geophys. Res. Lett. 40, 1132–1137 (2013).
Fransson, A. et al. CO2-system development in young sea ice and CO2 gas exchange at the ice/air interface mediated by brine and frost flowers in Kongsfjorden, Spitsbergen. Ann. Glaciol. 56, 245–257 (2015).
Geilfus, N. X. et al. First estimates of the contribution of CaCO3 precipitation to the release of CO2 to the atmosphere during young sea ice growth. J. Geophys. Res. Ocean. 118, 244–255 (2013).
Brown, K. A. et al. Inorganic carbon system dynamics in landfast Arctic sea ice during the early-melt period. J. Geophys. Res. Ocean. 120, 3542–3566 (2015).
Damm, E., Rudels, B., Schauer, U., Mau, S. & Dieckmann, G. Methane excess in Arctic surface water- triggered by sea ice formation and melting. Sci. Rep. 5, 16179 (2015).
Kort, E. A. et al. Atmospheric observations of Arctic Ocean methane emissions up to 82° north. Nat. Geosci. 5, 318–321 (2012).
Tison, J.-L. Biogeochemical impact of snow cover and cyclonic intrusions on the winter weddell sea ice pack. J. Geophys. Res. Ocean. 122, 7291–7311 (2017).
AMAP Assessment 2015: Methane as an Arctic Climate Forcer (AMAP, 2015).
Zhou, J. et al. Physical and biogeochemical properties in landfast sea ice (Barrow, Alaska): insights on brine and gas dynamics across seasons. J. Geophys. Res. Ocean. 118, 3172–3189 (2013).
Levasseur, M. Impact of Arctic meltdown on the microbial cycling of sulphur. Nat. Geosci. 6, 691–700 (2013).
Hayashida, H. et al. Implications of sea-ice biogeochemistry for oceanic production and emissions of dimethyl sulfide in the Arctic. Biogeosciences 14, 3129–3155 (2017).
Abbatt, J. P. D. et al. Overview paper: new insights into aerosol and climate in the Arctic. Atmos. Chem. Phys. 19, 2527–2560 (2019).
Galindo, V. et al. Biological and physical processes influencing sea ice, under-ice algae, and dimethylsulfoniopropionate during spring in the Canadian Arctic Archipelago. J. Geophys. Res. Ocean. 119, 3746–3766 (2014).
Simpson, W. R. et al. Halogens and their role in polar boundary-layer ozone depletion. Atmos. Chem. Phys. 7, 4375–4418 (2007).
Jacobi, H.-W., Morin, S. & Bottenheim, J. W. Observation of widespread depletion of ozone in the springtime boundary layer of the central Arctic linked to mesoscale synoptic conditions. J. Geophys. Res. Atmos. 115, 17302 (2010).
Abbatt, J. P. D. et al. Halogen activation via interactions with environmental ice and snow in the polar lower troposphere and other regions. Atmos. Chem. Phys. 12, 6237–6271 (2012).
Frey, M. M. et al. First direct observation of sea salt aerosol production from blowing snow above sea ice. Atmos. Chem. Phys. 20, 2549–2578 (2020).
Tarasick, D. W. & Bottenheim, J. W. Surface ozone depletion episodes in the Arctic and Antarctic from historical ozonesonde records. Atmos. Chem. Phys. 2, 197–205 (2002).
Kiko, R., Kern, S., Kramer, M. & Mütze, H. Colonization of newly forming Arctic sea ice by meiofauna: a case study for the future Arctic? Polar Biol. 40, 1277–1288 (2017).
Steiner, N. & Stefels, J. Commentary on the outputs and future of Biogeochemical Exchange Processes at Sea-Ice Interfaces (BEPSII). Elem. Sci. Anth. 5, 81 (2017).
Echeveste, P., Agustí, S. & Dachs, J. Cell size dependent toxicity thresholds of polycyclic aromatic hydrocarbons to natural and cultured phytoplankton populations. Environ. Pollut. 158, 299–307 (2010).
Peeken, I. et al. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 9, 1505 (2018).
Obbard, R. W. et al. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Futur. 2, 315–320 (2014).
Steiner, N. S., Christian, J. R., Six, K. D., Yamamoto, A. & Yamamoto-Kawai, M. Future ocean acidification in the Canada Basin and surrounding Arctic Ocean from CMIP5 earth system models. J. Geophys. Res. Ocean. 119, 332–347 (2014).
Fransson, A. et al. Impact of sea-ice processes on the carbonate system and ocean acidification at the ice-water interface of the Amundsen Gulf, Arctic Ocean. J. Geophys. Res. Ocean. 118, 7001–7023 (2013).
Geilfus, N.-X. et al. Estimates of ikaite export from sea ice to the underlying seawater in a sea ice–seawater mesocosm. Cryosphere 10, 2173–2189 (2016).
Moreau, S. et al. Assessment of the sea-ice carbon pump: Insights from a three-dimensional ocean-sea-ice biogeochemical model (NEMO-LIM-PISCES). Elementa 4, 000122 (2016).
Acknowledgements
This Perspective is a product of the Biogeochemical Exchange Processes at Sea-Ice Interfaces (BEPSII) research community. This manuscript was first conceived at the Arctic Sea-Ice Change foresight workshop held in Davos, Switzerland, in June 2018 and is supported by the Euromarine Network.
Author information
Authors and Affiliations
Contributions
D.L., L.T., M. v.L., K.C., H.F., B.D., L.M. and J.S. led the design and the writing of the paper. G.C., F.F., N.S., M.V. and M.V. significantly contributed to the ‘Environmental conditions’ section. P.A., J.B., H.K., K.M., I.P., J.-M.R. and P.W. significantly contributed to the ‘Biota’ section. K.B., M.C., O.C., E.D., B.E., A.F., N.-X.G., C.J., E.J., M.K., S.M., D.N., N.S., J.-L.T. and F.v.d.L. significantly contributed to the ‘Gases’ section.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Jørgen Berge, Suhas Shetye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Source Data Fig. 2
Historical and ‘worst-case’ RCP8.5 scenario source data.
Rights and permissions
About this article
Cite this article
Lannuzel, D., Tedesco, L., van Leeuwe, M. et al. The future of Arctic sea-ice biogeochemistry and ice-associated ecosystems. Nat. Clim. Chang. 10, 983–992 (2020). https://doi.org/10.1038/s41558-020-00940-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-00940-4
This article is cited by
-
Under-ice observations by trawls and multi-frequency acoustics in the Central Arctic Ocean reveals abundance and composition of pelagic fauna
Scientific Reports (2023)
-
Marine ecosystem shifts with deglacial sea-ice loss inferred from ancient DNA shotgun sequencing
Nature Communications (2023)
-
Seasonal sea-ice in the Arctic’s last ice area during the Early Holocene
Communications Earth & Environment (2023)
-
Natural short-lived halogens exert an indirect cooling effect on climate
Nature (2023)
-
Plastic pollution in the Arctic
Nature Reviews Earth & Environment (2022)