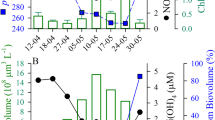
Abstract
Diatoms, large bloom-forming marine microorganisms, build frustules out of silicate, which ballasts the cells and aids their export to the deep ocean. This unique physiology forges an important link between the marine silicon and carbon cycles. However, the effect of ocean acidification on the silicification of diatoms is unclear. Here we show that diatom silicification strongly diminishes with increased acidity in a natural Antarctic community. Analyses of single cells from within the community reveal that the effect of reduced pH on silicification differs among taxa, with several species having significantly reduced silica incorporation at CO2 levels equivalent to those projected for 2100. These findings suggest that, before the end of this century, ocean acidification may influence the carbon and silicon cycle by both altering the composition of the diatom assemblages and reducing cell ballasting, which will probably alter vertical flux of these elements to the deep ocean.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support these findings are available from the Australian Antarctic Data Centre (https://doi.org/10.26179/5c3e745a9b071)69.
References
Khatiwala, S., Primeau, F. & Hall, T. Reconstruction of the history of anthropogenic CO2 concentrations in the ocean. Nature 462, 346–349 (2009).
Sabine, C. L. et al. The oceanic sink for anthropogenic CO2. Science 305, 367–371 (2004).
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005).
Riebesell, U. et al. Reduced calcification in marine plankton in response to increased atmospheric CO2. Nature 407, 634–637 (2000).
Fabry, V. J. Marine calcifiers in a high-CO2 ocean. Science 320, 1020–1022 (2008).
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (2009).
Levitan, I. et al. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Change Biol. 13, 1–8 (2007).
Tortell, P. et al. CO2 sensitivity of Southern Ocean phytoplankton. Geophys. Res. Lett. 35, L04605 (2008).
Wu, Y., Gao, K. & Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7, 2915–2923 (2010).
Schaum, E., Rost, B., Millar, A. J. & Collins, S. Variation in plastic responses of a globally distributed picoplankton species to ocean acidification. Nat. Clim. Change 3, 298–302 (2013).
Wu, Y., Campbell, D. A., Irwin, A. J., Suggett, D. J. & Finkel, Z. V. Ocean acidification enhances the growth rate of larger diatoms. Limnol. Oceanogr. 59, 1027–1034 (2014).
Dutkiewicz, S. et al. Impact of ocean acidification on the structure of future phytoplankton communities. Nat. Clim. Change 5, 1002–1006 (2015).
Mackey, K. R. M., Morris, J. J., Morel, F. M. M. & Kranz, S. A. Response of photosynthesis to ocean acidification. Oceanography 28, 74–91 (2015).
Riebesell, U., Gattuso, J.-P., Thingstad, T. F. & Meddleburg, J. J. Arctic ocean acidification: pelagic ecosystem and biogeochemical responses during a mesocosm study. Biogeosciences 10, 5619–5626 (2013).
Sala, M. M. et al. Contrasting effects of ocean acidification on the microbial food web under different trophic conditions. ICES J. Mar. Sci. 73, 670–679 (2015).
Burkhardt, S., Riebesell, U. & Zondervan, I. Effects of growth rate, CO2 concentration, and cell size on the stable carbon isotope fractionation in marine phytoplankton. Geochim. Cosmochim. Acta 63, 3729–3741 (1999).
Tortell, P. D., DiTullio, G., Sigman, D. M. & Morel, F. M. M. CO2 effects on taxonomic composition and nutrient utilization in an equatorial Pacific phytoplankton assemblage. Mar. Ecol. Prog. Ser. 236, 37–43 (2002).
Nelson, D. M., Treguer, P., Brzezinski, M. A., Leynaert, A. & Queguiner, B. Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycles 9, 359–372 (1995).
Martin-Jezequel, V., Hildebrand, M. & Brzezinski, M. A. Silicon metabolism in diatoms: implications for growth. J. Phycol. 36, 821–840 (2000).
Smetacek, V. Diatoms and the ocean carbon cycle. Protist 150, 25–32 (1999).
Hamm, C. E. et al. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421, 841–843 (2003).
Buesseler, K. O. The decoupling of production and particle export in the surface ocean. Glob. Biogeochem. Cycles 12, 297–310 (1998).
Dugdale, R. C. & Wilkerson, F. P. Silicate regulation of new production in the equatorial Pacific upwelling. Nature 391, 270–273 (1998).
Agusti, S. et al. Ubiquitous healthy diatoms in the deep sea confirm deep carbon injection by the biological pump. Nat. Commun. 6, 7608 (2015).
Tréguer, P. et al. Influence of diatom diversity on the ocean biological carbon pump. Nat. Geosci. 11, 27–37 (2018).
Baines, S. B., Twining, B. S., Brzezinski, M. A., Nelson, D. M. & Fisher, N. S. Causes and biogeochemical implications of regional differences in silicification of marine diatoms. Glob. Biogeochem. Cycles 24, GB4031 (2010).
Bach, L. T. et al. Effects of elevated CO2 on a natural diatom community in the subtropical NE Atlantic. Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00075 (2019).
Tatters, A. O. et al. Short- and long-term conditioning of a temperate marine diatom community to acidification and warming. Phil. Trans. R. Soc. B 368, 20120437 (2013).
Davidson, A. T. et al. Enhanced CO2 concentrations change the structure of Antarctic marine microbial communities. Mar. Ecol. Prog. Ser. 552, 93–113 (2016).
Schulz, K. G. et al. Phytoplankton blooms at increasing levels of atmospheric carbon dioxide: experimental evidence for negative effects on prymnesiophytes and positive on small picoeukaryotes. Front. Mar. Sci. 4, 64 (2017).
Hancock, A. M. et al. Ocean acidification changes the structure of an Antarctic coastal protistan community. Biogeosci. Discuss. 15, 2393–2410 (2018).
Riebesell, U. & Tortell, P. D. in Ocean Acidification (eds Gattuso, J.-P. & Hansson, L.) 99–121 (Oxford Univ. Press, 2011).
Deppeler, S. et al. Ocean acidification of a coastal Antarctic marine microbial community reveals a critical threshold for CO2 tolerance in phytoplankton productivity. Biogeosciences 15, 209 (2018).
Westwood, K. et al. Ocean acidification impacts primary and bacterial production in Antarctic coastal waters during austral summer. J. Exp. Mar. Biol. Ecol. 498, 46–60 (2018).
Milligan, A. J. & Morel, F. M. A proton buffering role for silica in diatoms. Science 297, 1848–1850 (2002).
Hervé, V. et al. Multiparametric analyses reveal the pH-dependence of silicon biomineralization in diatoms. PLoS ONE 7, e46722 (2012).
Milligan, A. J., Varela, D. E., Brzezinski, M. A. & Morel, F. M. M. Dynamics of silicon metabolism and silicon isotopic discrimination in a marine diatom as a function of \(p_{\mathrm {CO}_2}\). Limnol. Oceanogr. 49, 322–329 (2004).
Sugie, K. & Yoshimura, T. Effects of high CO2 levels on the ecophysiology of the diatom Thalassiosira weissflogii differ depending on the iron nutritional status. ICES J. Mar. Sci. 73, 680–692 (2016).
Riebesell, U. & Gattuso, J.-P. Lessons learned from ocean acidification research. Nat. Clim. Change 5, 12–14 (2015).
Havenhand, J., Dupont, S. & Quinn, G. P. in Guide to Best Practices for Ocean Acidification Research and Data Reporting (eds Riebesell, U. et al.) 67–80 (Publications Office of the European Union, 2010).
Roden, N. P., Shadwick, E. H., Tilbrook, B. & Trull, T. W. Annual cycle of carbonate chemistry and decadal change in coastal Prydz Bay, East Antarctica. Mar. Chem. 155, 135–147 (2013).
Dutkiewicz, S., Scott, J. R. & Follows, M. J. Winners and losers: ecological and biogeochemical changes in a warming ocean. Glob. Biogeochem. Cycles 27, 463–477 (2013).
Miklasz, K. A. & Denny, M. W. Diatom sinking speeds: improved predictions and insight from a modified Stoke’s law. Limnol. Oceanogr. 55, 2513–2525 (2010).
McNair, H. M., Brzezinski, M. A., Till, C. P. & Krause, J. W. Taxon‐specific contributions to silica production in natural diatom assemblages. Limnol. Oceanogr. 63, 1056–1075 (2018).
Shi, D., Xu, Y., Hopkinson, B. M. & Morel, F. M. M. Effect of ocean acidification on iron availability to marine phytoplankton. Science 327, 676–679 (2010).
Villareal, T. A., Altabet, M. A. & Culver-Rymsza, K. Nitrogen transport by vertically migrating diatom mats in the North Pacific Ocean. Nature 363, 709–712 (1993).
Durkin, C. A. et al. Frustule-related gene transcription and the influence of diatom community composition on silica precipitation in an iron-limited environment. Limnol. Oceanogr. 57, 1619–1633 (2012).
McNeil, B. & Matear, R. Southern Ocean acidification: a tipping point at 450-ppm atmospheric CO2. Proc. Natl Acad. Sci. USA 105, 18860–18864 (2008).
Assmy, P. et al. Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic Circumpolar Current. Proc. Natl Acad. Sci. USA 110, 20633–20638 (2013).
Kang, S. & Fryxell, G. Fragilariopsis cylindrus (Grunow) Krieger: the most abundant diatom in water column assemblages of Antarctic marginal ice-edge zones. Polar Biol. 12, 609–627 (1992).
Matsumoto, K., Sarmiento, J. L. & Brzezinski, M. A. Silicic acid leakage from the Southern Ocean: a possible explanation for glacial atmospheric \(p_{\mathrm {CO}_2}\). Glob. Biogeochem. Cycles 16, 5–1 (2002).
Boyd, P. W. Physiology and iron modulate diverse responses of diatoms to a warming Southern Ocean. Nat. Clim. Change 9, 148–152 (2019).
Boyd, P. W., Lennartz, S. T., Glover, D. M. & Doney, S. C. Biological ramifications of climate-change-mediated oceanic multi-stressors. Nat. Clim. Change 5, 71–79 (2014).
Petrou, K. et al. Southern Ocean phytoplankton physiology in a changing climate. J. Plant Physiol. 203, 135–150 (2016).
Boyd, P. W. et al. Physiological responses of a Southern Ocean diatom to complex future ocean conditions. Nat. Clim. Change 6, 207–213 (2016).
Deppeler, S. L. & Davidson, A. T. Southern Ocean phytoplankton in a changing climate. Front. Mar. Sci. 4, 40 (2017).
Dickson, A. G., Sabine, C. L. & Christian, J. R. Guide to Best Practices for Ocean CO 2 Measurements (PICES Special Publication, North Pacific Marine Science Organization, 2007).
Dickson, A. G. Standards for ocean measurements. Oceanography 23, 34–47 (2010).
Leblanc, K. & Hutchins, D. A. New applications of a biogenic silica deposition fluorophore in the study of oceanic diatoms. Limnol. Oceanogr. Methods 3, 462–476 (2005).
Shimizu, K., Del Amo, Y., Brzezinski, M. A., Stucky, G. D. & Morse, D. E. A novel fluorescent silica tracer for biological silicification studies. Chem. Biol. 8, 1051–1060 (2001).
McNair, H. M., Brzezinski, M. A. & Krause, J. W. Quantifying diatom silicification with the fluorescent dye, PDMPO. Limnol. Oceanogr. Methods 13, 587–599 (2015).
Baker, K. G. et al. Thermal performance curves of functional traits aid understanding of thermally induced changes in diatom-mediated biogeochemical fluxes. Front. Mar. Sci. 3, 44 (2016).
Parambath, M. et al. The nature of the silicaphilic fluorescence of PDMPO. Phys. Chem. Chem. Phys. 18, 5938–5948 (2016).
Strickland, J. D. H. & Parsons, T. R. A Practical Handbook of Seawater Analysis (Fisheries Research Board of Canada, 1968).
Nelson, D. M. et al. Particulate matter and nutrient distributions in the ice-edge zone of the Weddell Sea: relationship to hydrography during late summer. Deep Sea Res. Pt A 36, 191–209 (1989).
Schneider, C. A., Raspnad, W. S. & Eliceiri, K. W. NIH image to imageJ: 25 years of image analysis. Nat. Methods 9, 671–679 (2012).
Kreyling, J. et al. To replicate, or not to replicate—that is the question: how to tackle nonlinear responses in ecological experiments. Ecol. Lett. 21, 1629–1638 (2018).
Cottingham, K. L., Lennon, J. T. & Brown, B. L. Knowing when to draw the line: designing more informative ecological experiments. Front. Ecol. Environ. 3, 145–152 (2005).
Petrou, K. Antarctic Diatom Silicification Diminishes Under Ocean Acidification (Australian Antarctic Data Centre, 2019); https://doi.org/10.26179/5c3e745a9b071.
Acknowledgements
The work was supported by Australian Antarctic Science project AAS 4026 from the Australian Antarctic Division (AAD); samples were imported under permit no. IP13019928. We are grateful to AAD technical support for their assistance and support in designing and equipping the mesocosm facility and to the Davis Station expeditioners in the summer of 2014/2015.
Author information
Authors and Affiliations
Contributions
K.P. conceptualized the study. K.P., K.G.B., D.A.N., A.M.H., K.G.S. and A.T.D. carried out the investigations. K.P. and K.G.B. developed the methodology. K.P. and D.A.N. conducted the formal analysis and visualization. K.P. prepared the original draft. K.P., K.G.B., D.A.N., A.M.H., K.G.S. and A.T.D. reviewed and edited it. K.P. and A.T.D. obtained the funding and provided resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Climate Change thanks Phillipp Assmy, Paul Treguer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Tables 1–6 and references.
Rights and permissions
About this article
Cite this article
Petrou, K., Baker, K.G., Nielsen, D.A. et al. Acidification diminishes diatom silica production in the Southern Ocean. Nat. Clim. Chang. 9, 781–786 (2019). https://doi.org/10.1038/s41558-019-0557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-019-0557-y
This article is cited by
-
Contrasting patterns in pH variability in the Arabian Sea and Bay of Bengal
Environmental Science and Pollution Research (2024)
-
Plastic responses lead to increased neurotoxin production in the diatom Pseudo-nitzschia under ocean warming and acidification
The ISME Journal (2023)
-
A deep-learning estimate of the decadal trends in the Southern Ocean carbon storage
Nature Communications (2022)
-
Sinking diatoms trap silicon in deep seawater of acidified oceans
Nature (2022)
-
Enhanced silica export in a future ocean triggers global diatom decline
Nature (2022)