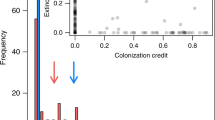
Abstract
Anthropogenic climate change is predicted to cause many extinctions worldwide1. Although species endemic to islands or archipelagos have high conservation value and are vulnerable to human impacts2,3, there has been no global analysis of climate-driven extinction risk focused on island endemics. Here, we use conifers as a model system to assess extinction risk among island endemics under climate projections for 2070. We employ the emerging technique of combining native and non-native occurrence data to model climatic conditions under which each species can sustain a population4,5,6,7 and also incorporate horticultural data to model the broader range of conditions that allow short-term survival. Our projections indicate that some species will retain suitable climatic conditions, some will experience conditions completely precluding survival and others will experience intermediate-risk conditions that lead to population decline and eventual extinction. Based on different climate change models, we report island size thresholds of 400 to 20,000 km2, below which extinction risks increase. These patterns are driven by correlations among island area and the breadth of species’ realized, fundamental and tolerance niches. Notably, realized and fundamental niche breadth are positively correlated. Our results highlight management interventions needed to protect species from climate-driven extinction across islands of different sizes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Some herbaria and consortia we queried for species occurrence data do not allow dissemination of their data beyond the user, so we are unable to publish the portion of our dataset derived from these online sources. However, these data are publicly available for download, so we provide information on where to access data from each herbarium and consortium in the references. For full details on herbarium consortia, including lists of individual participating herbaria whose data we used, see Supplementary Note 2. We also provide in the Supplementary Data a version of our species occurrence dataset that contains only the data derived from personal communication and our primary literature search. CHELSA climate data are publicly available at http://www.chelsa-climate.org and at the Dryad Digital Repository at https://doi.org/10.5061/dryad.kd1d4.
References
Urban, M. C. Accelerating extinction risk from climate change. Science 348, 571–573 (2015).
Kier, G. et al. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl Acad. Sci. USA 106, 9322–9327 (2009).
Fordham, D. A. & Brook, B. W. Why tropical island endemics are acutely susceptible to global change. Biodivers. Conserv. 19, 329–342 (2010).
Sax, D. F., Early, R. & Bellemare, J. Niche syndromes, species extinction risks, and management under climate change. Trends Ecol. Evol. 28, 517–523 (2013).
Booth, T. H. Using a global botanic gardens database to help assess the capabilities of rare eucalypt species to cope with climate change. Int. For. Rev. 17, 259–268 (2015).
Bocsi, T. et al. Plants’ native distributions do not reflect climatic tolerance. Divers. Distrib. 22, 615–624 (2016).
Perret, D. L., Leslie, A. B. & Sax, D. F. Naturalized distributions show that climatic disequilibrium is structured by niche size in pines (Pinus L.). Glob. Ecol. Biogeogr. 28, 429–441 (2019).
Harter, D. E. V. et al. Impacts of global climate change on the floras of oceanic islands: projections, implications and current knowledge. Perspect. Plant Ecol. Syst. 17, 160–183 (2015).
Ferreira, M. T. et al. Effects of climate change on the distribution of indigenous species in oceanic islands (Azores). Clim. Change 138, 603–615 (2016).
Fortini, L. et al. A Landscape-Based Assessment of Climate Change Vulnerability for all Native Plants Technical Report No. HCSU-044 (University of Hawai’i, 2013).
Booth, T. H. Assessing species climatic requirements beyond the realized niche: some lessons mainly from tree species distribution modeling. Clim. Change 145, 259–271 (2017).
Guisan, A., Petitpierre, B., Broennimann, O., Daehler, C. & Kueffer, C. Unifying niche shift studies: insights from biological invasions. Trends Ecol. Evol. 29, 260–269 (2014).
Early, R. & Sax, D. F. Climatic niche shifts between species’ native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Glob. Ecol. Biogeogr. 23, 1356–1365 (2014).
Faurby, S. & Araujo, M. B. Anthropogenic range contractions bias species climate change forecasts. Nat. Clim. Change 8, 252–256 (2018).
Li, Y. M., Liu, X., Li, X. P., Petitpierre, B. & Guisan, A. Residence time, expansion toward the equator in the invaded range and native range size matter to climatic niche shifts in non-native species. Glob. Ecol. Biogeogr. 23, 1094–1104 (2014).
Bush, A. et al. Truncation of thermal tolerance niches among Australian plants. Glob. Ecol. Biogeogr. 27, 22–31 (2018).
Kambach, S. et al. Of niches and distributions: range size increases with niche breadth both globally and regionally but regional estimates poorly relate to global estimates. Ecography 41, 1–11 (2018).
Hill, M. P., Gallardo, B. & Terblanche, J. S. A global assessment of climatic niche shifts and human influence in insect invasions. Glob. Ecol. Biogeogr. 26, 679–689 (2017).
Farjon, A. & Filer, D. An Atlas of the World’s Conifers: An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status (Brill, 2013).
Richardson, D. M. & Rejmanek, M. Conifers as invasive aliens: a global survey and predictive framework. Divers. Distrib. 10, 321–331 (2004).
Karger, D. N. et al. Data descriptor: climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 170122 (2017).
Petitpierre, B., Broennimann, O., Kueffer, C., Daehler, C. & Guisan, A. Selecting predictors to maximize the transferability of species distribution models: lessons from cross-continental plant invasions. Glob. Ecol. Biogeogr. 26, 275–287 (2017).
Yu, F. Y. et al. Climatic niche breadth can explain variation in geographical range size of alpine and subalpine plants. Int. J. Geogr. Inf. Sci. 31, 190–212 (2017).
Thuiller, W., Lavorel, S. & Araujo, M. B. Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob. Ecol. Biogeogr. 14, 347–357 (2005).
Dullinger, S. et al. Extinction debt of high-mountain plants under twenty-first century climate change. Nat. Clim. Change 2, 619–622 (2012).
Cheke, A. & Hume, J. Lost Land of the Dodo (Yale Univ. Press, 2008).
Holt, R. D., Barfield, M. & Gomulkiewicz, R. in Species Invasions: Insights Into Ecology, Evolution, and Biogeography (eds Sax, D. F. et al.) 259–290 (Sinauer Associates Inc., 2005).
Skelly, D. K. et al. Evolutionary responses to climate change. Conserv. Biol. 21, 1353–1355 (2007).
Lesk, C., Coffel, E., D’Amato, A. W., Dodds, K. & Horton, R. Threats to North American forests from southern pine beetle with warming winters. Nat. Clim. Change 7, 713–717 (2017).
Dobrowski, S. Z. A climatic basis for microrefugia: the influence of terrain on climate. Glob. Change Biol. 17, 1022–1035 (2011).
Farjon, A. Conifers of the World BRAHMS Database (RBG Kew, accessed 30 October 2017); https://herbaria.plants.ox.ac.uk/bol/conifers
SERNEC Data Portal (SouthEast Regional Network of Expertise and Collections, accessed 9 September 2017); http://sernecportal.org/portal/index.php
CNH Data Portal (Consortium of Northeastern Herbaria, accessed 20 September 2017); www.neherbaria.org
Consortium of Pacific Northwest Herbaria Specimen Database (CPNWH) (Consortium of Pacific Northwest Herbaria, accessed 23 September 2017); www.pnwherbaria.org
The C. V. Starr Virtual Herbarium (New York Botanical Garden, accessed 26 September 2017); http://sweetgum.nybg.org/science/vh/
Botany Collection Search: Information Provided with the Permission of the National Museum of Natural History (Smithsonian Institution, accessed 2 October 2017); https://collections.nmnh.si.edu/search/botany/
Tropicos: Botanical Information System at the Missouri Botanical Garden (Missouri Botanical Garden, accessed 7 October 2017); www.tropicos.org
Harvard University Herbarium Catalogue (HUH, accessed 11 October 2017); http://kiki.huh.harvard.edu/databases/specimen_index.html
Consortium of California Herbaria (CCH) Catalogue (accessed 14 October 2017). Consortium of California Herbaria; http://ucjeps.berkeley.edu/consortium
University of Michigan Herbarium (accessed 16 October 2017). University of Michigan Herbarium; https://michiganflora.net/specimen-search.aspx
The Australasian Virtual Herbarium (Council of Heads of Australasian Herbaria, accessed 16 October 2017); http://avh.chah.org.au
Allan Herbarium Specimen Data (Manaaki Whenua/Landcare Research, accessed 30 October 2017); http://scd.landcareresearch.co.nz
Botany Collections Online. (Auckland Museum, accessed 3 November 2017). Auckland Museum; http://www.aucklandmuseum.com/discover/collections-online
NZFRI Online Dataset (Scion, accessed 6 November 2017). Scion; nzfri.scionresearch.com
Botanical Database of Southern Africa (BODATSA) (SANBI. South African National Biodiversity Institute, accessed 9 November 2017). South African National Biodiversity Institute; http://newposa.sanbi.org/
The Herbarium Catalogue, Royal Botanic Gardens, Kew (Kew, accessed 10 November 2017). Kew; http://www.kew.org/herbcat
Royal Botanic Garden Edinburgh Herbarium Catalogue (RBGE, accessed 15 November 2017). Royal Botanic Garden Edinburgh; http://data.rbge.org.uk/search/herbarium/
Muséum National d’Histoire Naturelle Catalogue (MNHN, accessed 20 November 2017). Muséum National d’Histoire Naturelle; https://science.mnhn.fr/institution/mnhn/collection/p/item/search/form
Swedish Naturhistoriska Riksmuseet Catalogue (NRM, accessed 28 November 2017). Swedish Naturhistoriska Riksmuseet; http://herbarium.nrm.se/
Baard, J. A. & Kraaij, T. Alien flora of the Garden Route National Park, South Africa. S. Afr. J. Bot. 94, 51–63 (2014).
Badalamenti, E., LaMantia, T. & Pasta, S. Primo caso di naturalizzazione di Pinus canariensis C. Sm. (Pinaceae) per la Sicilia e prima stazione di Acacia cyclops G. Don (Fabaceae) sull’isola maggiore. Nat. Sicil. 37, 497–503 (2013).
Borges, P. A. V. et al. (eds) A List of the Terrestrial and Marine Biota from the Azores (Princípia, Cascais, 2010).
Celesti-Grapow, L. et al. Inventory of the non-native flora of Italy. Plant Biosyst. 143, 386–440 (2009).
Cronk, Q. C. B. The past and present vegetation of St. Helena. J. Biogeogr. 16, 47–64 (1989).
Daehler, C. C. & Baker, R. F. New records of naturalized and naturalizing plants around Lyon Arboretum, Manoa Valley, O’ahu. Occas. Pap. Bernice Pauahi Bish. Mus. 87, 3–18 (2006).
Firsov, G. A. & Byalt, V. V. Review of woody exotic species producing self-seeding in St. Petersburg (Russia). Russ. J. Biol. Invasions 7, 84–104 (2016).
Galasso, G. et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. 152, 179–303 (2018).
Hall, C. R. et al. Plant records. Watsonia 17, 183–198 (1988).
Heenan, P. B., de Lange, P. J., Cameron, E. K. & Champion, P. D. Checklist of dicotyledons, gymnosperms, and pteridophytes naturalised or casual in New Zealand: additional records 1999–2000. N. Z. J. Bot. 40, 155–174 (2002).
Henderson, L. & Wilson, J. R. U. Changes in the composition and distribution of alien plants in South Africa: an update from the Southern African Plant Invaders Atlas. Bothalia 47, a2172 (2017).
Hill, K. D. in Flora of Australia https://go.nature.com/30o2ZpJ (Australian Biological Resources Study, Department of the Environment and Energy, 2017).
Kafle, G. & Savillo, I. T. Present status of Ramsar sites in Nepal. Int. J. Biodivers. Conserv. 1, 146–150 (2009).
Kondo, T. & Tsuyuzaki, S. Natural regeneration patterns of the introduced larch, Larix kaempferi (Pinaceae), on the volcano Mount Koma, northern Japan. Divers. Distrib. 5, 223–233 (1999).
Lambdon, P. & Darlow, A. Botanical Survey of Ascension Island and St. Helena (Royal Society for the Protection of Birds, 2008).
Lau, A. & Frohlich, D. New plant records for the Hawaiian Islands 2011–2012. Occas. Pap. Bernice Pauahi Bish. Mus. 114, 5–16 (2013).
de Lima, R. F. Land-Use Management and the Conservation of Endemic Species in the Island of São Tomé. PhD thesis, Lancaster Univ. (2012).
de Lima, R. F. et al. Distribution and habitat associations of the critically endangered bird species of São Tomé Island (Gulf of Guinea). Bird Conserv. Int. 27, 455–469 (2017).
Morita, S. et al. Unusually high levels of bio-available phosphate in the soils of Ogasawara Islands, Japan: putative influence of seabirds. Geoderma 160, 155–164 (2010).
Oppenheimer, H. L. The spread of gymnosperms on Maui: a neglected element of the modern Hawaiian flora. Occas. Pap. Bernice Pauahi Bish. Mus. 68, 19–23 (2002).
Oppenheimer, H. L. New plant records from Moloka’i, Läna’i, Maui, and Hawai’i for 2006. Occas. Pap. Bernice Pauahi Bish. Mus. 96, 17–34 (2007).
Oppenheimer, H. L. New Hawaiian plant records for 2007. Occas. Pap. Bernice Pauahi Bish. Mus. 100, 22–38 (2008).
Rouget, M., Richardson, D. M., Milton, S. J. & Polakow, D. Predicting invasion dynamics of four alien Pinus species in a highly fragmented semi-arid shrubland in South Africa. Plant Ecol. 152, 79–92 (2001).
Shimizu, Y. & Tabata, H. Invasion of Pinus lutchuensis and its influence on the native forest on a Pacific Island. J. Biogeogr. 12, 195–207 (1985).
Singh, R. S., Rahlan, P. K. & Singh, S. P. Phytosociological and population structure of oak-mixed conifer forest in a part of Kumaun Himalaya. Environ. Ecol. 5, 475–487 (1987).
Webb, C. J., Sykes, W. R. & Garnock-Jones, P. J. Naturalised Pteridophytes, Gymnosperms, Dicotyledons. Flora of New Zealand Vol. 4 (Botany Division, DSIR, 1988).
Woo, E. The Role of Plant-Bird Interactions in the Invasion of Juniperus bermudiana in Hawaii: Integrating Experiments, Behavior, and Models (State University of New York at Stony Brook, ProQuest Dissertations Publishing, 2008).
Beck, J., Ballesteros-Mejia, L., Nagel, P. & Kitching, I. J. Online solutions and the ‘Wallacean shortfall’: what does GBIF contribute to our knowledge of species’ ranges? Divers. Distrib. 19, 1043–1050 (2013).
Broennimann, O. et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 21, 481–497 (2012).
Qiao, H. J., Escobar, L. E., Saupe, E. E., Ji, L. Q. & Soberon, J. A cautionary note on the use of hypervolume kernel density estimators in ecological niche modelling. Glob. Ecol. Biogeogr. 26, 1066–1070 (2017).
McSweeney, C. F., Jones, R. G., Lee, R. W. & Rowell, D. P. Selecting CMIP5 GCMs for downscaling over multiple regions. Clim. Dynam. 44, 3237–3260 (2014).
R Core Team R: A Language and Environment for Statistical computing (R Foundation for Statistical Computing, 2017); https://www.R-project.org
Hijmans, R. J. raster: Geographic Data Analysis and Modeling. R version 2.6-7 https://CRAN.R-project.org/package=raster(2017).
QGIS Development Team QGIS Geographic Information System (Open Source Geospatial Foundation Project, 2017); http://qgis.osgeo.org
Leutner, B. & Horning, N. RStoolbox: tools for remote sensing data analysis. R version 0.1.10 https://CRAN.R-project.org/package=RStoolbox (2017).
Calenge, C. The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Model. 197, 516–519 (2006).
Bivand, R. & Rundel C. rgeos: interface to geometry engine - open source (‘GEOS’). R version 0.3-26 https://CRAN.R-project.org/package=rgeos (2017).
Spiess, A. N. qpcR: modelling and analysis of real-time PCR data. R version 1.4-0 https://CRAN.R-project.org/package=qpcR (2014).
Mazerolle, M. J. AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R version 2.1-1 https://cran.r-project.org/package=AICcmodavg (2017).
Jackman, S. pscl: classes and methods for R developed in the Political Science Computational Laboratory. R version 1.5.2 https://github.com/atahk/pscl/ (2017).
Henningsen, A. & Hamann, J. D. systemfit: a package for estimating systems of simultaneous equations in R. J. Stat. Softw. 23, 1–40 (2007).
Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in general parametric models. Biom. J. 50, 346–363 (2008).
Jurasinski, G. & Retzer, V. simba: a collection of functions for similarity analysis of vegetation data. R version 0.3-5 https://CRAN.R-project.org/package=simba (2012).
Becker, R. A., Wilks, A. R., Brownrigg, R., Minka, T. P. & Deckmyn, A. maps: draw geographical maps. R version 3.2.0 https://CRAN.R-project.org/package=maps (2017).
Acknowledgements
This manuscript benefitted from financial support from the Institute at Brown for Environment and Society. Species occurrence data were provided by L. Celesti-Grapow, R. de Lima, L. Henderson, G. Firsov, A. Khmarik, S. Peccenini, R. Wagensommer, J. Baard, D. Luscombe, B. Chaves, G. Dyer and P. Normandy. Many botanists and horticulturalists provided helpful replies to our requests for additional information regarding non-native species occurrences in our dataset.
Author information
Authors and Affiliations
Contributions
K.C.R. and D.F.S. designed the study and wrote the manuscript. K.C.R. collected the data and performed the analyses. D.L.P. helped design the data collection approach, contributed to some analyses, assisted in interpretation and contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Climate Change thanks Carsten Külheim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Tables 1–2, methods and Supplementary Notes 1 and 2.
Supplementary Data 1
Species occurrence data derived from personal communication and our primary literature search.
Rights and permissions
About this article
Cite this article
Rosenblad, K.C., Perret, D.L. & Sax, D.F. Niche syndromes reveal climate-driven extinction threat to island endemic conifers. Nat. Clim. Chang. 9, 627–631 (2019). https://doi.org/10.1038/s41558-019-0530-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-019-0530-9