Abstract
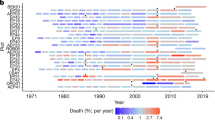
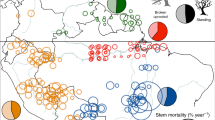
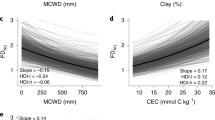
Tree mortality appears to be increasing in moist tropical forests1, with potentially important implications for global carbon and water cycles2. Little is known about the drivers of tree mortality in these diverse forests, partly because long-term data are lacking3. The relative importance of climatic factors and species functional traits as drivers of tropical tree mortality are evaluated using a unique dataset in which the survival of over 1,000 rainforest canopy trees from over 200 species has been monitored monthly over five decades in the Central Amazon. We found that drought, as well as heat, storms and extreme rainy years, increase tree mortality for at least two years after the climatic event. Specific functional groups (pioneers, softwoods and evergreens) had especially high mortality during extreme years. These results suggest that predicted climate change will lead to higher tree mortality rates, especially for short-lived species, which may result in faster carbon sequestration but lower carbon storage of tropical forests.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The mortality data that support the findings of this study are available from the corresponding author on reasonable request. Climate data from Reserva Floresta Adolpho Ducke are available from LBA (http://lba2.inpa.gov.br/) on request. Tree trait data obtained for the current study from the TRY database and Global Wood Density Database can be requested from www.try-db.org and https://datadryad.org//.
Code availability
The R code used to produce the Cox hazard model is available as Supplementary Code.
References
Mcdowell, N. et al. Drivers and mechanisms of tree mortality in moist tropical forests. New Phytol. 219, 851–869 (2018).
Reichstein, M. et al. Climate extremes and the carbon cycle. Nature 500, 287–295 (2013).
Brienen, R. J. W. et al. Long-term decline of the Amazon carbon sink. Nature 519, 344–348 (2015).
Adams, H. D. et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 1, 1285–1291 (2017).
Mori, S. A. & Becker, P. Flooding affects survival of lecythidaceae in terra firme forest near Manaus, Brazil. Biotropica 23, 87–90 (1991).
Allen, C. D. et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 259, 660–684 (2010).
Nelson, B. W. et al. Forest disturbance by large blowdowns in the Brazilian Amazon. Ecology 75, 853–858 (1994).
Leitold, V. et al. El Niño drought increased canopy turnover in Amazon forests. New Phytol. 219, 959–971 (2018).
Bennett, A. C., Mcdowell, N. G., Allen, C. D. & Anderson-teixeira, K. J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 1, 15139 (2015).
Laurance, W. F. et al. Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature 428, 171–175 (2004).
Van der Sande, M. T. et al. Old‐growth neotropical forests are shifting in species and trait composition. Ecol. Monogr. 86, 228–243 (2016).
Chao, K. J. et al. Growth and wood density predict tree mortality in Amazon forests. J. Ecol. 96, 281–292 (2008).
Malhi, Y. et al. Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172 (2008).
Gloor, M. et al. Intensification of the Amazon hydrological cycle over the last two decades. Geophys. Res. Lett. 40, 1729–1733 (2013).
Phillips, O. et al. Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347 (2009).
Phillips, O. et al. Drought–mortality relationships for tropical forests. New Phytol. 187, 631–646 (2010).
Condit, R., Hubbell, S. P. & Foster, R. B. Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol. Monogr 65, 419–439 (1995).
Nakagawa, M. et al. Impact of severe drought associated with the 1997–1998 El Niño in a tropical forest in Sarawak. J. Trop. Ecol. 16, 355–367 (2000).
Williamson, G. B. et al. Amazonian tree mortality during the 1997 El Nino drought. Conserv. Biol. 14, 1538–1542 (2000).
Negrón-Juárez, R. I. et al. Widespread Amazon forest tree mortality from a single cross-basin squall line event. Geophys. Res. Lett. 37, L16701 (2010).
Brokaw, N. V. L., Pickett, S. T. A. & White, P. S. The Ecology of Natural Disturbance and Patch Dynamics. Academic Press, New York, USA. (1985).
Marengo, J. A. et al. The drought of Amazonia in 2005. J. Clim. 21, 495–516 (2008).
Negrón-Juárez, R. et al. Windthrow variability in central Amazonia. Atmosphere 8, 28 (2017).
Chambers, J. Q. et al. The steady-state mosaic of disturbance and succession across an old-growth Central Amazon forest landscape. Proc. Natl Acad. Sci. USA 110, 3949–3954 (2013).
Mueller, R. C. et al. Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts. J. Ecol. 93, 1085–1093 (2005).
Van Gelder, H. A., Poorter, L. & Sterck, F. J. Wood mechanics, allometry, and life-history variation in a tropical rain forest tree community. New Phytol. 171, 367–378 (2006).
Sevanto, S., Mcdowell, N. G., Dickman, L. T., Pangle, R. & Pockman, W. T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 37, 153–161 (2014).
Wright, S. J. et al. Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91, 3664–3674 (2010).
Fontes, C. G., Chambers, J. Q. & Higuchi, N. Revealing the causes and temporal distribution of tree mortality in Central Amazonia. For. Ecol. Manage. 424, 177–183 (2018).
Clark, D. B., Clark, D. A. & Oberbauer, S. F. Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Glob. Change Biol. 16, 747–759 (2010).
Anderegg, W. R. L. et al. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 8, 367–371 (2015).
Marengo, J. A., Tomasella, J., Soares, W. R., Alves, L. M. & Nobre, C. A. Extreme climatic events in the Amazon Basin. Theor. Appl. Climatol. 107, 73–85 (2012).
Cailleret, M. et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Change Biol. 23, 1675–1690 (2017).
Nepstad, D. C. et al. Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology 88, 2259–2269 (2007).
Laurance, W. F. et al. An Amazonian rainforest and its fragments as a laboratory of global change. Biol. Rev. 93, 223–247 (2017).
Poorter, L., Castilho, C. V., Schietti, J., Oliveira, R. S. & Costa, F. R. C. Can traits predict individual growth performance? A test in a hyperdiverse tropical forest. New Phytol. 219, 109–121 (2018).
Ribeiro, J. E. L. S. et al. Flora da Reserva Ducke: Guia de Identificação das Plantas Vasculares de uma Floresta de Terra-Firme na Amazônia Central (INPA-DFID, 1999).
Chauvel, A., Lucas, Y. & Boulet, R. On the genesis of the soil mantle of the region of Manaus, Central Amazonia, Brazil. Experientia 43, 234–241 (1987).
Peel, M. C., Finlayson, B. L. & McMahon, T. A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. Discuss. 4, 439–473 (2007).
Da Cruz Alencar, J., de Almeida, R. A. & Fernandes, N. P. Fenologia de especies florestais em floresta tropical úmida de terra firme na Amazônia Central.Acta Amaz. 9, 163–198 (1979).
Phillips, O. L. et al. Pattern and process in Amazon tree turnover, 1976–2001. Phil. Trans. R. Soc. B 359, 381–407 (2004).
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009).
Zanne, A. E. et al. Global Wood Density Database (Dryad Digital Repository, 2009).
Nogueira, E. M., Nelson, B. W. & Fearnside, P. M. Wood density in dense forest in Central Amazonia, Brazil. For. Ecol. Manage. 208, 261–286 (2005).
Ferraz, I. D. K., Leal Filho, N., Imakawa, A. M., Varela, V. P. & Piña-Rodrigues, F. C. M. Características básicas para um agrupamento ecológico preliminar de espécies madeireiras da floresta de terra firme da Amazônia Central. Acta Amaz. 34, 621–633 (2004).
Dias, D. P. & Marenco, R. A. Tree growth, wood and bark water content of 28 Amazonian tree species in response to variations in rainfall and wood density. iForest 9, 445–451 (2016).
Singh, K. P. & Kushwaha, C. P. Deciduousness in tropical trees and its potential as indicator of climate change: a review. Ecol. Indic. 69, 699–706 (2016).
Ishida, A. et al. Contrasting seasonal leaf habits of canopy trees between tropical dry-deciduous and evergreen forests in Thailand. Tree Physiol. 26, 643–656 (2006).
Kattge, J. et al. TRY—a global database of plant traits. Glob. Change Biol. 17, 2905–2935 (2011).
Lohbeck, M. et al. Successional changes in functional composition contrast for dry and wet tropical forest. Ecology 94, 1211–1216 (2013).
Amaral, D. D., Viera, I. C. G., Salomão, R. P., Almeida, S. S de. & Jardim, M. A. G. Checklist da flora arb¢rea de remanescentes florestais da regiao metropolitana de Belém, Pará, Brasil.Bol. Mus. Para. Emilio Goeldi Ciênc. Nat 4, 231–289 (2009).
Lima, R. B. D. A. et al. Sucessão ecológica de um trecho de floresta ombrófila densa de terras baixas, Carauari, Amazonas. Pesqui. Florest. Bras 2011, 161–172 (2011).
Malhi, Y. & Wright, J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Phil. Trans. R. Soc. B 359, 311–329 (2004).
Marengo, J. A. & Espinoza, J. C. Extreme seasonal droughts and floods in Amazonia: causes, trends and impacts. Int. J. Climatol. 36, 1033–1050 (2016).
Coelho, C. A. S. et al. Climate diagnostics of three major drought events in the Amazon and illustrations of their seasonal precipitation predictions. Meteorol. Appl. 19, 237–255 (2012).
Condit, R. et al. Tropical forest dynamics across a rainfall gradient and the impact of an El Niño dry season. J. Trop. Ecol. 20, 51–72 (2004).
Schöngart, J. & Junk, W. J. Forecasting the flood-pulse in Central Amazonia by ENSO-indices. J. Hydrol. 335, 124–132 (2007).
Feldpausch, T. R. et al. Amazon forest response to repeated droughts. Glob. Biogeochem. Cycles 30, 964–982 (2016).
Asner, G. P., Townsend, A. R. & Braswell, B. H. Satellite observation of El Niño effects on Amazon forest phenology and productivity. Geophys. Res. Lett. 27, 981–984 (2000).
Saleska, S. R., Didan, K., Huete, A. R. & da Rocha, H. R. Amazon forests green-up during 2005 drought. Science 318, 612 (2007).
Wright, S. J. & Calderon, O. Seasonal, El Niño and longer term changes in flower and seed production in a moist tropical forest. Ecol. Lett. 9, 35–44 (2006).
Chapman, C. A., Valenta, K., Bonnell, T. R., Brown, K. A. & Chapman, L. J. Solar radiation and ENSO predict fruiting phenology patterns in a 15-year record from Kibale National Park, Uganda. Biotropica 50, 384–395 (2018).
Barlow, J. & Peres, C. A. Ecological responses to El Nino-induced surface fires in central Brazilian Amazonia: management implications for flammable tropical forests. Phil. Trans. R. Soc. 359, 367–380 (2004).
Flores, B. M., Piedade, M. T. F. & Nelson, B. W. Fire disturbance in Amazonian blackwater floodplain forests. Plant Ecol. Divers. 7, 319–327 (2014).
Niño 3 SST Index (National Oceanic and Atmospheric Administration, NOAA, 2017); http://www.esrl.noaa.gov/psd/gcos_wgsp/Timeseries/Nino3/
Grimm, A. M., Barros, V. & Doyle, M. Climate variability in southern South America associated with El Niño and La Niña events. J. Clim. 13, 35–58 (2000).
North Atlantic Oscillation (NAO) (National Centers for Environmental Information, NOAA, 2017); https://www.ncdc.noaa.gov/teleconnections/nao/
Sheil, D., Burslem, D. F. R. P. & Alder, D. The interpretation and misinterpretation of mortality rate measures. J. Ecol. 83, 331–333 (1995).
Therneau, T. A package for survival analysis in S. R package v.2.37-7 (CRAN, 2015); https://cran.r-project.org/web/packages/survival/index.html
Therneau, T. M. & Grambsch, P. M. in Modeling Survival data: Extending the Cox Model 39–77 (Springer, 2000).
Venables, W. N. & Ripley, B. D. in Modern Applied Statistics with S 271–300 (Springer, 2002).
Acknowledgements
The authors thank the researchers V. Campbell de Araújo and J. da Cruz Alencar for implementing the phenological research and selecting the trees at the beginning of the monitoring. Numerous grants have financed more than 50 years of research. We are grateful to the Coordination of Technology and Innovation (COTEI) and Forestry Research Group of Amazon Species at the National Institute of Amazonian Research for providing data, and to the field technicians J. Maciel, M. Azevedo, L. Reis, E. Nascimento and T. Nascimento for conducting field work. We also appreciate contributions from colleagues at the National Institute of Amazonian Research, the Forest Ecology and Management Group at Wageningen University and Research, and the Herbarium at the Royal Botanic Gardens, Kew. We are grateful for the contributions of B. Nelson and S. Saleska during the revision process. I.A. was supported by the PDSE programme (88881.1349/84/2016-01), from the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES).
Author information
Authors and Affiliations
Contributions
I.A., D.N., A.B., L.P. and F.C. planned the study. A.B. and I.A. collected the data. I.A., D.N. and E.P. organized the datasets. L.H., I.A. and L.P. conducted the analyses. All authors contributed to writing the manuscript.
Corresponding author
Additional information
Journal peer review information: Nature Climate Change thanks Emanuel Gloor, S. Joseph Wright and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1, Supplementary Figures 1–7 and Supplementary Code
Rights and permissions
About this article
Cite this article
Aleixo, I., Norris, D., Hemerik, L. et al. Amazonian rainforest tree mortality driven by climate and functional traits. Nat. Clim. Chang. 9, 384–388 (2019). https://doi.org/10.1038/s41558-019-0458-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-019-0458-0
This article is cited by
-
Enhanced future vegetation growth with elevated carbon dioxide concentrations could increase fire activity
Communications Earth & Environment (2024)
-
Divergent hydraulic strategies of two deciduous tree species to deal with drought in the Brazilian semi-arid region
Trees (2024)
-
Impacts of a severe storm on carbon accumulation in coarse woody debris within a secondary Atlantic Forest fragment in Brazil
Environmental Monitoring and Assessment (2024)
-
Does climate change alter the nutrient trends of Cedrela fissilis Vell. trees in the southern Brazilian Amazon?
Ecological Processes (2023)
-
Amazon windthrow disturbances are likely to increase with storm frequency under global warming
Nature Communications (2023)