Abstract
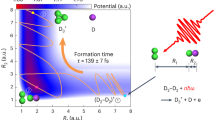
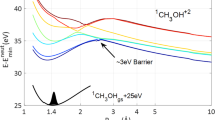
The H2–H2 molecular dimer is of fundamental importance in the study of chemical interactions because of its unique bonding properties and its ability to model more complex systems. The trihydrogen cation H3+ is also a key intermediate in a range of chemical processes in interstellar environments, such as the formation of various organic molecules and early stars. However, the unexpected high abundance of H3+ in molecular clouds remains challenging to explain. Here using near-infrared, femtosecond laser pulses and coincidence momentum imaging, we find that the dominant channel after photoionization of a deuterium molecular dimer (D2–D2) is the ejection of a deuterium atom within a few hundred femtoseconds, leading to the formation of D3+. The formation mechanism is supported and well-reproduced by ab initio molecular dynamics simulations. This pathway of D3+ formation from ultracold D2–D2 gas may provide insights into the high abundance of H3+ in the interstellar medium.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study and the raw data for all the figures have been uploaded to Figshare36 at https://doi.org/10.6084/m9.figshare.21509769. Source data are provided with this paper.
Code availability
The code for the ab initio molecular dynamics simulation is available from the corresponding author upon reasonable request.
References
Oka, T. Interstellar H3+. Proc. Natl Acad. Sci. USA 103, 12235–12242 (2006).
Geballe, T. R. & Oka, T. A key molecular ion in the universe and in the laboratory. Science 312, 1610–1612 (2006).
Oka, T. Interstellar H3+. Chem. Rev. 113, 8738–8761 (2013).
Geballe, T. R. & Oka, T. Detection of H3+ in interstellar space. Nature 384, 334–335 (1996).
McCall, B. J., Geballe, T. R., Hinkle, K. H. & Oka, T. Detection of H3+ in the diffuse interstellar medium toward Cygnus OB2 No. 12. Science 279, 1910–1913 (1998).
Thomson, J. J. XXVI. Rays of positive electricity. London Edinburgh Dublin Philos. Mag. J. Sci. 21, 225–249 (1911).
Hogness, T. R. & Lunn, E. G. The ionization of nitrogen by electron impact as interpreted by positive ray analysis. Phys. Rev. 26, 786–793 (1925).
Eland, J. H. D. The origin of primary H3+ ions in mass spectra. Rapid Commun. Mass Spectrom. 10, 1560–1562 (1996).
De, S., Rajput, J., Roy, A., Ghosh, P. N. & Safvan, C. P. Formation of H3+ due to intramolecular bond rearrangement in doubly charged methanol. Phys. Rev. Lett. 97, 213201 (2006).
Zhang, Y. et al. Formation of H3+ from ethane dication induced by electron impact. Commun. Chem. 3, 160 (2020).
Wang, E., Ren, X. & Dorn, A. Role of the environment in quenching the production of H3+ from dicationic clusters of methanol. Phys. Rev. Lett. 126, 103402 (2021).
Furukawa, Y., Hoshina, K., Yamanouchi, K. & Nakano, H. Ejection of triatomic hydrogen molecular ion from methanol in intense laser fields. Chem. Phys. Lett. 414, 117–121 (2005).
Livshits, E., Luzon, I., Gope, K., Baer, R. & Strasser, D. Time-resolving the ultrafast H2 roaming chemistry and H3+ formation using extreme-ultraviolet pulses. Commun. Chem. 3, 49 (2020).
Okino, T. et al. Coincidence momentum imaging of ultrafast hydrogen migration in methanol and its isotopomers in intense laser fields. Chem. Phys. Lett. 423, 220–224 (2006).
Hoshina, K., Furukawa, Y., Okino, T. & Yamanouchi, K. Efficient ejection of H3+ from hydrocarbon molecules induced by ultrashort intense laser fields. J. Chem. Phys. 129, 104302 (2008).
Kraus, P. M. et al. Unusual mechanism for H3+ formation from ethane as obtained by femtosecond laser pulse ionization and quantum chemical calculations. J. Chem. Phys. 134, 114302 (2011).
Kotsina, N., Kaziannis, S. & Kosmidis, C. Hydrogen migration in methanol studied under asymmetric fs laser irradiation. Chem. Phys. Lett. 604, 27–32 (2014).
Ekanayake, N. et al. Substituent effects on H3+ formation via H2 roaming mechanisms from organic molecules under strong-field photodissociation. J. Chem. Phys. 149, 244310 (2018).
Ekanayake, N. et al. Mechanisms and time-resolved dynamics for trihydrogen cation (H3+) formation from organic molecules in strong laser fields. Sci. Rep. 7, 4703 (2017).
Ekanayake, N. et al. H2 roaming chemistry and the formation of H3+ from organic molecules in strong laser fields. Nat. Commun. 9, 5186 (2018).
Ando, T., Iwasaki, A. & Yamanouchi, K. Strong-field Fourier transform vibrational spectroscopy of D2+ using few-cycle near-infrared laser pulses. Phys. Rev. Lett. 120, 263002 (2018).
Alghabra, M. S. et al. Anomalous formation of trihydrogen cations from water on nanoparticles. Nat. Commun. 12, 3839 (2021).
Knee, L. B. G. & Brunt, C. M. A massive cloud of cold atomic hydrogen in the outer Galaxy. Nature 412, 308–310 (2001).
Duley, W. W. The formation of H2 by H-atom reaction with grain surfaces. Mon. Not. R. Astron. Soc. 279, 591–594 (1996).
Fletcher, L. N., Gustafsson, M. & Orton, G. S. Hydrogen dimers in giant-planet infrared spectra. Astrophys. J. Suppl. Ser. 235, 24 (2018).
Mckellar, A. R. W. Possible identification of sharp features in the Voyager far-infrared spectra of Jupiter and Saturn. Can. J. Phys. 62, 760–763 (1984).
Frommhold, L., Samuelson, R. & Birnbaum, G. Hydrogen dimer structures in the far-infrared spectra of Jupiter and Saturn. Astrophys. J. 283, L79–L82 (1984).
Gibb, B. C. Chemistry of the sky god. Nat. Chem. 12, 974–976 (2020).
Ullrich, J. et al. Recoil-ion and electron momentum spectroscopy: reaction-microscopes. Rep. Prog. Phys. 66, 1463–1545 (2003).
Bucksbaum, P. et al. Softening of the H2+ molecular bond in intense laser fields. Phys. Rev. Lett. 64, 1883–1886 (1990).
Sussman, B. J., Townsend, D., Ivanov, M. Y. & Stolow, A. Dynamic Stark control of photochemical processes. Science 314, 278–281 (2006).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, 2016).
Schlegel, H. B. et al. Ab initio molecular dynamics: propagating the density matrix with Gaussian orbitals. J. Chem. Phys. 114, 9758–9763 (2001).
Iyengar, S. S. et al. Ab initio molecular dynamics: propagating the density matrix with Gaussian orbitals. II. Generalizations based on mass-weighting, idempotency, energy conservation and choice of initial conditions. J. Chem. Phys. 115, 10291–10302 (2001).
Schlegel, H. B. et al. Ab initio molecular dynamics: propagating the density matrix with Gaussian orbitals. III. Comparison with Born-Oppenheimer dynamics. J. Chem. Phys. 117, 8694–8704 (2002).
Mi, Y. et al. Observation and dynamic control of a new pathway of H3+ formation (figshare, 2022); https://doi.org/10.6084/m9.figshare.21509769
Acknowledgements
We thank A. R. W. McKellar, A. Stolow, P. Bunker and T. Pfeifer for fruitful discussions. We acknowledge support from the Joint Centre for Extreme Photonics. Y.M. acknowledges support from the Deutsche Forschungsgemeinschaft (German Research Foundation) grant no. MI 2434/1-1 (Y.M.). E.W. is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, grant no. XDB34020000 (E.W.), and the Alexander von Humboldt Foundation. Financial support from the National Science and Engineering Research Council Discovery (grant no. RGPIN-2020-05858, A.S.) and from the US Air Force Office of Scientific Research (grant no. FA9550-16-1-0109, P.C.) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Y.M. designed and conducted the experiments. E.W. performed molecular dynamics simulations. Z.D., A.Y.N. and A.S. assisted with the experiments. Y.M. and A.S. analysed the experimental data. Y.M., E.W. and A.S. wrote the manuscript with contributions from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Marcos Dantus and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparisons of the simulated center of mass distance as a function of time for the H2 and D2 dimer.
(a), (b), and (c) correspond to the T-shape geometry, parallel geometry and X-shape geometry of the H2 (red curves) and D2 (blue curves) dimers, respectively. The results show that all initial configurations lead to the formation of H3+ or D3+. The molecule begins to dissociate once the trajectory shows a linear increase of the center of mass distance as a function of time. These results indicate that the H2 dimer dissociates faster than the D2 dimer.
Extended Data Fig. 2 Simulated trajectories of H3+ formation as a function of time.
The simulated center of mass distance of H3+ + H is shown for 493 trajectories, out of a total of 593 simulated trajectories for H2–H2. The branching ratio for this channel is 83.1%, which is very close to that of the D3+ + D channel (89.2%).
Extended Data Fig. 3 Simulated time-dependent H3+ yield.
The simulated data is obtained by projecting the trajectories with a center-of-mass distance larger than 4.2 Å onto the time axis, which excludes most of the oscillation structures. The red curve represents a fit to the data using the growth function Y(t) = a(1-e−t/τ) + Y0, where a and Y0 represent the amplitude and the offset of the H3+ yield, respectively, and τ is the formation time of H3+. By using this fit function, we find the formation time of H3+ is 169. 5 fs.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, discussion and Table 1.
Supplementary Video 1
The formation process of D3+ for a parallel-aligned D2 dimer.
Supplementary Video 2
The formation process of D3+ for a T-shape D2 dimer.
Supplementary Video 3
The formation process of D3+ for an X-shape D2 dimer.
Source data
Source Data Fig. 1
Calculated PES and the one-dimensional and two-dimensional trajectory simulations for D2 dimers.
Source Data Fig. 2
Statistical source data of the time-of-flight spectrum.
Source Data Fig. 3
Statistical source data of the photoion-photoion coincidence spectrum.
Source Data Fig. 4
Statistical source data of the yields of different pathways.
Source Data Extended Data Fig./Table 1
Calculated trajectory simulation (one-dimensional) for H2 and D2 dimers.
Source Data Extended Data Fig./Table 2
Calculated two-dimensional trajectory simulation for H2 dimers.
Source Data Extended Data Fig./Table 3
Simulated time-dependent H3+ yield.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mi, Y., Wang, E., Dube, Z. et al. D3+ formation through photoionization of the molecular D2–D2 dimer. Nat. Chem. 15, 1224–1228 (2023). https://doi.org/10.1038/s41557-023-01231-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01231-z
This article is cited by
-
Ultrafast photoinduced C-H bond formation from two small inorganic molecules
Nature Communications (2024)
-
Insights into ultrafast H3+ formation provide a glimpse into primordial chemistry
Nature Chemistry (2023)
-
H2 formation via non-Born-Oppenheimer hydrogen migration in photoionized ethane
Nature Communications (2023)