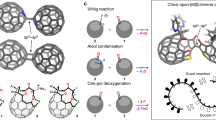
Abstract
The regioselective functionalization of C60 remains challenging, while the enantioselective functionalization of C60 is difficult to explore due to the need for complex chiral tethers or arduous chromatography. Metal–organic cages have served as masks to effect the regioselective functionalization of C60. However, it is difficult to control the stereochemistry of the resulting fullerene adducts through this method. Here we report a means of defining up to six stereocentres on C60, achieving enantioselective fullerene functionalization. This method involves the use of a metal–organic cage built from a chiral formylpyridine. Fullerenes hosted within the cavity of the cage can be converted into a series of C60 adducts through chemo-, regio- and stereo-selective Diels–Alder reactions with the edges of the cage. The chiral formylpyridine ultimately dictates the stereochemistry of these chiral fullerene adducts without being incorporated into them. Such chiral fullerene adducts may become useful in devices requiring circularly polarized light manipulation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are included within the Article and its Supplementary Information, and are also available from the corresponding author on request. Crystallographic data for the structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre, under the deposition numbers 24124135 (ΛΛΛΛ-1), 24124134 (ΔΛΛΛ-C60·2) and 24124133 (PCBM·2′). Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Percec, V. et al. Steric communication of chiral information observed in dendronized polyacetylenes. J. Am. Chem. Soc. 128, 16365–16372 (2006).
Clayden, J., Lund, A., Vallverdú, L. & Helliwell, M. Ultra-remote stereocontrol by conformational communication of information along a carbon chain. Nature 431, 966–971 (2004).
Yashima, E. et al. Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 116, 13752–13990 (2016).
Tsiamantas, C. et al. Selective dynamic assembly of disulfide macrocyclic helical foldamers with remote communication of handedness. Angew. Chem. Int. Ed. 55, 6848–6852 (2016).
Eelkema, R. et al. Nanomotor rotates microscale objects. Nature 440, 163–163 (2006).
Han, B. et al. Asymmetric organocatalysis: an enabling technology for medicinal chemistry. Chem. Soc. Rev. 50, 1522–1586 (2021).
Zhao, C. et al. Chiral amide directed assembly of a diastereo- and enantiopure supramolecular host and its application to enantioselective catalysis of neutral substrates. J. Am. Chem. Soc. 135, 18802–18805 (2013).
Pan, M., Wu, K., Zhang, J.-H. & Su, C.-Y. Chiral metal–organic cages/containers (MOCs): From structural and stereochemical design to applications. Coord. Chem. Rev. 378, 333–349 (2019).
Argent, S. P., Riis-Johannessen, T., Jeffery, J. C., Harding, L. P. & Ward, M. D. Diastereoselective formation and optical activity of an M4L6 cage complex. Chem. Commun. 4647–4649 (2005)..
Castilla, A. M. et al. High-fidelity stereochemical memory in a FeII4L4 tetrahedral capsule. J. Am. Chem. Soc. 135, 17999–18006 (2013).
Nishioka, Y., Yamaguchi, T., Kawano, M. & Fujita, M. Asymmetric [2 + 2] olefin cross photoaddition in a self-assembled host with remote chiral auxiliaries. J. Am. Chem. Soc. 130, 8160–8161 (2008).
Mislow, K. & Siegel, J. Stereoisomerism and local chirality. J. Am. Chem. Soc. 106, 3319–3328 (1984).
Howlader, P., Zangrando, E. & Mukherjee, P. S. Self-assembly of enantiopure Pd12 tetrahedral homochiral nanocages with tetrazole linkers and chiral recognition. J. Am. Chem. Soc. 142, 9070–9078 (2020).
Xuan, W., Zhang, M., Liu, Y., Chen, Z. & Cui, Y. A chiral quadruple-stranded helicate cage for enantioselective recognition and separation. J. Am. Chem. Soc. 134, 6904–6907 (2012).
Brown, C. J., Bergman, R. G. & Raymond, K. N. Enantioselective catalysis of the aza-Cope rearrangement by a chiral supramolecular assembly. J. Am. Chem. Soc. 131, 17530–17531 (2009).
Mahata, K., Frischmann, P. D. & Würthner, F. Giant electroactive M4L6 tetrahedral host self-assembled with Fe(II) vertices and perylene bisimide dye edges. J. Am. Chem. Soc. 135, 15656–15661 (2013).
Kishi, N., Li, Z., Yoza, K., Akita, M. & Yoshizawa, M. An M2L4 molecular capsule with an anthracene shell: encapsulation of large guests up to 1 nm. J. Am. Chem. Soc. 133, 11438–11441 (2011).
Purba, P. C., Maity, M., Bhattacharyya, S. & Mukherjee, P. S. A self-assembled palladium(II) barrel for binding of fullerenes and photosensitization ability of the fullerene-encapsulated barrel. Angew. Chem. Int. Ed. 60, 14109–14116 (2021).
Nakamura, T., Ube, H., Miyake, R. & Shionoya, M. A C60-templated tetrameric porphyrin barrel complex via zinc-mediated self-assembly utilizing labile capping ligands. J. Am. Chem. Soc. 135, 18790–18793 (2013).
Huang, N. et al. Tailor-made pyrazolide-based metal–organic frameworks for selective catalysis. J. Am. Chem. Soc. 140, 6383–6390 (2018).
Fuertes-Espinosa, C. et al. Supramolecular fullerene sponges as catalytic masks for regioselective functionalization of C60. Chem 6, 169–186 (2020).
Ubasart, E. et al. A three-shell supramolecular complex enables the symmetry-mismatched chemo- and regioselective bis-functionalization of C60. Nat. Chem. 13, 420–427 (2021).
Chen, B., Holstein, J. J., Horiuchi, S., Hiller, W. G. & Clever, G. H. Pd(II) coordination sphere engineering: pyridine cages, quinoline bowls, and heteroleptic pills binding one or two fullerenes. J. Am. Chem. Soc. 141, 8907–8913 (2019).
Leonhardt, V., Fimmel, S., Krause, A.-M. & Beuerle, F. A covalent organic cage compound acting as a supramolecular shadow mask for the regioselective functionalization of C60. Chem. Sci. 11, 8409–8415 (2020).
Nishimura, T. et al. Macromolecular helicity induction on a poly(phenylacetylene) with C2-symmetric chiral [60]fullerene-bisadducts. J. Am. Chem. Soc. 126, 11711–11717 (2004).
Bianco, A. et al. Synthesis, chiroptical properties, and configurational assignment of fulleroproline derivatives and peptides. J. Am. Chem. Soc. 118, 4072–4080 (1996).
Shi, W. et al. Fullerene desymmetrization as a means to achieve single-enantiomer electron acceptors with maximized chiroptical responsiveness. Adv. Mater. 33, 2004115 (2021).
Fuertes-Espinosa, C., Pujals, M. & Ribas, X. Supramolecular purification and regioselective functionalization of fullerenes and endohedral metallofullerenes. Chem 6, 3219–3262 (2020).
Thilgen, C. & Diederich, F. Structural aspects of fullerene chemistry – a journey through fullerene chirality. Chem. Rev. 106, 5049–5135 (2006).
Riala, M. & Chronakis, N. A facile access to enantiomerically pure [60]fullerene bisadducts with the inherently chiral trans-3 addition pattern. Org. Lett. 13, 2844–2847 (2011).
Filippone, S., Maroto, E. E., Martín-Domenech, Á., Suarez, M. & Martín, N. An efficient approach to chiral fullerene derivatives by catalytic enantioselective 1,3-dipolar cycloadditions. Nat. Chem. 1, 578–582 (2009).
Djojo, F. & Hirsch, A. Synthesis and chiroptical properties of enantiomerically pure bis- and trisadducts of C60 with an inherent chiral addition pattern. Chem. Eur. J. 4, 344–356 (1998).
Evans, D. A., Ennis, M. D. & Mathre, D. J. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of alpha-substituted carboxylic acid derivatives. J. Am. Chem. Soc. 104, 1737–1739 (1982).
Bordoli, R. J. & Goldup, S. M. An efficient approach to mechanically planar chiral rotaxanes. J. Am. Chem. Soc. 136, 4817–4820 (2014).
Naaman, R., Paltiel, Y. & Waldeck, D. H. Chiral molecules and the electron spin. Nat. Rev. Chem. 3, 250–260 (2019).
Meng, W., Clegg, J. K., Thoburn, J. D. & Nitschke, J. R. Controlling the transmission of stereochemical information through space in terphenyl-edged Fe4L6 cages. J. Am. Chem. Soc. 133, 13652–13660 (2011).
Ousaka, N. et al. Efficient long-range stereochemical communication and cooperative effects in self-assembled Fe4L6 cages. J. Am. Chem. Soc. 134, 15528–15537 (2012).
Ronson, T. K., Pilgrim, B. S. & Nitschke, J. R. Pathway-dependent post-assembly modification of an anthracene-edged MII4L6 tetrahedron. J. Am. Chem. Soc. 138, 10417–10420 (2016).
Knof, U. & von Zelewsky, A. Predetermined chirality at metal centers. Angew. Chem. Int. Ed. 38, 302–322 (1999).
Guerra, S., Schillinger, F., Sigwalt, D., Holler, M. & Nierengarten, J.-F. Synthesis of optically pure [60]fullerene e,e,e-tris adducts. Chem. Commun. 49, 4752–4754 (2013).
Xiao, Z., Geng, X., He, D., Jia, X. & Ding, L. Development of isomer-free fullerene bisadducts for efficient polymer solar cells. Energy Environ. Sci. 9, 2114–2121 (2016).
Köhler, A. & Bässler, H. in Electronic Processes in Organic Semiconductors I–XIII (eds A. Köhler and H. Bässler) (John Wiley & Sons, 2015); https://doi.org/10.1002/9783527685172.fmatter
Naaman, R. & Waldeck, D. H. Spintronics and chirality: spin selectivity in electron transport through chiral molecules. Annu. Rev. Phys. Chem. 66, 263–281 (2015).
Bain, C. D. & Whitesides, G. M. Molecular-level control over surface order in self-assembled monolayer films of thiols on gold. Science 240, 62–63 (1988).
Yang, Y., da Costa, R. C., Fuchter, M. J. & Campbell, A. J. Circularly polarized light detection by a chiral organic semiconductor transistor. Nat. Photonics 7, 634–638 (2013).
O’Neill, M. & Kelly, S. M. Ordered materials for organic electronics and photonics. Adv. Mater. 23, 566–584 (2011).
Tarzia, A. & Jelfs, K. E. Unlocking the computational design of metal–organic cages. Chem. Commun. 58, 3717–3730 (2022).
Nguyen, T. D., Ehrenfreund, E. & Vardeny, Z. V. Spin-polarized light-emitting diode based on an organic bipolar spin valve. Science 337, 204–209 (2012).
Kim, Y.-H. et al. Chiral-induced spin selectivity enables a room-temperature spin light-emitting diode. Science 371, 1129–1133 (2021).
Stewart, J. J. P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 13, 1173–1213 (2007).
SCIGRESS version FJ 2.6 (EU 3.1.9) build 5996.8255.20141202 (Fujitsu Limited, 2013).
Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC, EP/P027067/1) and the European Research Council (695009). Z.L. acknowledges the Cambridge Trust and China Scholarship Council for PhD funding. A.W.H. is the recipient of an Astex Pharmaceuticals Sustaining Innovation Post-Doctoral Award. S.F. acknowledges funding from the Engineering and Physical Sciences Research Council (EPSRC, UK) through an EPSRC Doctoral Prize Fellowship. We thank Diamond Light Source for beamtime on Beamline I19 (CY21497). We also thank the Yusuf Hamied Department of Chemistry NMR facility for characterization data and C. Fuertes-Espinosa for helpful discussions.
Author information
Authors and Affiliations
Contributions
Z.L, T.K.R and J.R.N. conceived the study and wrote the manuscript. Z.L. performed the synthetic work with assistance from A.W.H. The X-ray data were collected by T.K.R. who also refined the structures. N.V. and A.M. performed chiral HPLC studies on the fullerene adducts. S.F. carried out the transient absorption measurements. Z.L led the project overall. All the authors contributed to the manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Celedonio Álvarez, Timothy Barendt and Xavi Ribas for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–111.
Supplementary Data 1
Crystallographic data for ΛΛΛΛ-1; (CCDC reference 24124135).
Supplementary Data 2
Crystallographic data for ΔΛΛΛ-C60·2; (CCDC reference 24124134).
Supplementary Data 3
Crystallographic data for PCBM·2′; (CCDC reference 24124133).
Supplementary Data 4
Statistical Source data for Supplementary Information.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Z., Ronson, T.K., Heard, A.W. et al. Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage. Nat. Chem. 15, 405–412 (2023). https://doi.org/10.1038/s41557-022-01103-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01103-y
This article is cited by
-
Complexation-driven assembly of imine-linked helical receptors showing adaptive folding and temperature-dependent guest selection
Nature Communications (2024)
-
Stereo-control on Lanthanide Triple-stranded Helicates Toward Enhanced Enantioselective Sensing
Chemical Research in Chinese Universities (2024)
-
Enantiocontrolled macrocyclization by encapsulation of substrates in chiral capsules
Nature Synthesis (2023)