Abstract
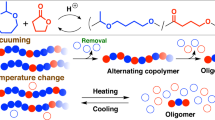
The existing catalyst/initiator systems and methodologies used for the synthesis of polymers can access only a few cyclic polymers composed entirely of a single monomer type, and the synthesis of such authentic cyclic polar vinyl polymers (acrylics) devoid of any foreign motifs remains a challenge. Here we report that a tethered B-P-B trifunctional, intramolecular frustrated Lewis pair catalyst enables the synthesis of an authentic cyclic acrylic polymer, cyclic poly(γ-methyl-α-methylene-γ-butyrolactone) (c-PMMBL), from the bio-based monomer MMBL. Detailed studies have revealed an initiation and propagation mechanism through pairwise monomer enchainment enabled by the cooperative and synergistic initiator/catalyst sites of the trifunctional catalyst. We propose that macrocyclic intermediates and transition states comprising two catalyst molecules are involved in the catalyst-regulated ring expansion and eventual cyclization, forming authentic c-PMMBL rings and concurrently regenerating the catalyst. The cyclic topology of the c-PMMBL polymers imparts an ~50 °C higher onset decomposition temperature and a much narrower degradation window compared with their linear counterparts of similar molecular weight and dispersity, while maintaining high chemical recyclability.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Full experimental details and the data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Bielawski, C. W., Benitez, D. & Grubbs, R. H. An “endless” route to cyclic polymers. Science 297, 2041–2044 (2002).
Hong, M. & Chen, E. Y.-X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat. Chem. 8, 42–49 (2016).
Haque, F. M. & Grayson, S. M. The synthesis, properties and potential applications of cyclic polymers. Nat. Chem. 12, 433–444 (2020).
Endo, K. Synthesis and properties of cyclic polymers. Adv. Polym. Sci. 217, 121–183 (2008).
Zhu, J.-B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Kammiyada, H., Ouchi, M. & Sawamoto, M. A study on physical properties of cyclic poly(vinyl ether)s synthesized via ring-expansion cationic polymerization. Macromolecules 50, 841–848 (2017).
Kricheldorf, H. R. Cyclic polymers: synthetic strategies and physical properties. J. Polym. Sci. A 48, 251–284 (2010).
Ziebarth, J. D. et al. Comparison of critical adsorption points of ring polymers with linear polymers. Macromolecules 49, 8780–8788 (2016).
Gambino, T., Martínez de Ilarduya, A., Alegría, A. & Barroso-Bujans, F. Dielectric relaxations in poly(glycidyl phenyl ether): effects of microstructure and cyclic topology. Macromolecules 49, 1060–1069 (2016).
Doi, Y. et al. Melt rheology of ring polystyrenes with ultrahigh purity. Macromolecules 48, 3140–3147 (2015).
Pasquino, R. et al. Viscosity of ring polymer melts. ACS Macro Lett. 2, 874–878 (2013).
Roland, C. D., Li, H., Abboud, K. A., Wagener, K. B. & Veige, A. S. Cyclic polymers from alkynes. Nat. Chem. 8, 791–796 (2016).
Yamamoto, T. & Tezuka, Y. Cyclic polymers revealing topology effects upon self-assemblies, dynamics and responses. Soft Matter 11, 7458–7468 (2015).
Tu, X.-Y., Liu, M.-Z. & Wei, H. Recent progress on cyclic polymers: synthesis, bioproperties, and biomedical applications. J. Polym. Sci. A: 54, 1447–1458 (2016).
Williams, R. J., Dove, A. P. & O’Reilly, R. K. Self-assembly of cyclic polymers. Polym. Chem. 6, 2998–3008 (2015).
Liénard, R., De Winter, J. & Coulembier, O. Cyclic polymers: advances in their synthesis, properties, and biomedical applications. J. Polym. Sci. 58, 1481–1502 (2020).
Romio, M. et al. Topological polymer chemistry enters materials science: expanding the applicability of cyclic polymers. ACS Macro Lett. 9, 1024–1033 (2020).
Zhang, K., Lackey, M. A., Cui, J. & Tew, G. N. Gels based on cyclic polymers. J. Am. Chem. Soc. 133, 4140–4148 (2011).
Chen, B., Jerger, K., Fréchet, J. M. J. & Szoka, F. C. Jr The influence of polymer topology on pharmacokinetics: differences between cyclic and linear PEGylated poly(acrylic acid) comb polymers. J. Control. Release 140, 203–209 (2009).
Wang, T.-W. & Golder, M. R. Advancing macromolecular hoop construction: recent developments in synthetic cyclic polymer chemistry. Polym. Chem. 12, 958–969 (2021).
Josse, T., De Winter, J., Gerbaux, P. & Coulembier, O. Cyclic polymers by ring-closure strategies. Angew. Chem. Int. Ed. 55, 13944–13958 (2016).
Chang, Y. A. & Waymouth, R. M. Recent progress on the synthesis of cyclic polymers via ring-expansion strategies. J. Polym. Sci. A 55, 2892–2902 (2017).
Sarkar, S. et al. An OCO3− trianionic pincer tungsten(VI) alkylidyne: rational design of a highly active alkyne polymerization catalyst. J. Am. Chem. Soc. 134, 4509–4512 (2012).
Miao, Z. et al. Cyclic polyacetylene. Nat. Chem. 13, 792–799 (2021).
Wang, T.-W., Huang, P.-R., Chow, J. L., Kaminsky, W. & Golder, M. R. A cyclic ruthenium benzylidene initiator platform enhances reactivity for ring-expansion metathesis polymerization. J. Am. Chem. Soc. 143, 7314–7319 (2021).
Gonsales, S. A. et al. Highly tactic cyclic polynorbornene: stereoselective ring expansion metathesis polymerization of norbornene catalyzed by a new tethered tungsten-alkylidene catalyst. J. Am. Chem. Soc. 138, 4996–4999 (2016).
Reisberg, S. H., Hurley, H. J., Mathers, R. T., Tanski, J. M. & Getzler, Y. D. Y. L. Lactide cyclopolymerization kinetics, X-ray structure, and solution dynamics of (tBu-SalAmEE)Al and a cautionary tale of polymetalate formation. Macromolecules 46, 3273–3279 (2013).
Piedra-Arroni, E., Ladavière, C., Amgoune, A. & Bourissou, D. Ring-opening polymerization with Zn(C6F5)2-based Lewis pairs: original and efficient approach to cyclic polyesters. J. Am. Chem. Soc. 135, 13306–13309 (2013).
Brown, H. A. & Waymouth, R. M. Zwitterionic ring-opening polymerization for the synthesis of high molecular weight cyclic polymers. Acc. Chem. Res. 46, 2585–2596 (2013).
Li, X.-Q., Wang, B., Ji, H.-Y. & Li, Y.-S. Insights into the mechanism for ring-opening polymerization of lactide catalyzed by Zn(C6F5)2/organic superbase Lewis pairs. Catal. Sci. Technol. 6, 7763–7772 (2016).
Asenjo-Sanz, I., Veloso, A., Miranda, J. I., Pomposo, J. A. & Barroso-Bujans, F. Zwitterionic polymerization of glycidyl monomers to cyclic polyethers with B(C6F5)3. Polym. Chem. 5, 6905–6908 (2014).
Guo, L., Lahasky, S. H., Ghale, K. & Zhang, D. N-Heterocyclic carbene-mediated zwitterionic polymerization of N-substituted N-Carboxyanhydrides toward poly(α-peptoid)s: kinetic, mechanism, and architectural control. J. Am. Chem. Soc. 134, 9163–9171 (2012).
Zhang, Y., Miyake, G. M. & Chen, E. Y.-X. Alane-based classical and frustrated Lewis pairs in polymer synthesis: rapid polymerization of MMA and naturally renewable methylene butyrolactones into high-molecular-weight polymers. Angew. Chem. Int. Ed. 49, 10158–10162 (2010).
McGraw, M. L. & Chen, E. Y.-X. Lewis pair polymerization: perspective on a ten-year journey. Macromolecules 53, 6102–6122 (2020).
Hong, M., Chen, J. & Chen, E. Y.-X. Polymerization of polar monomers mediated by main-group Lewis acid–base pairs. Chem. Rev. 118, 10551–10616 (2018).
Chen, E. Y.-X. Polymerization by classical and frustrated Lewis pairs. Top. Curr. Chem. 334, 239–260 (2012).
Wan, Y., He, J., Zhang, Y. & Chen, E. Y.-X. One-step synthesis of lignin-based triblock copolymers as high-temperature and UV-blocking thermoplastic elastomers. Angew. Chem. Int. Ed. (2022). https://doi.org/10.1002/anie.202114946
Stephan, D. W. Frustrated Lewis pairs: from concept to catalysis. Acc. Chem. Res. 48, 306–316 (2015).
Stephan, D. W. & Erker, G. Frustrated Lewis pair chemistry: development and perspectives. Angew. Chem. Int. Ed. 54, 6400–6441 (2015).
Stephan, D. W. The broadening reach of frustrated Lewis pair chemistry. Science 354, aaf7229 (2016).
Kehr, G. & Erker, G. Frustrated Lewis pair chemistry: searching for new reactions. Chem. Rec. 17, 803–815 (2017).
Jupp, A. R. & Stephan, D. W. New directions for frustrated Lewis pair chemistry. Trends Chem. 1, 35–48 (2019).
Zhao, W., He, J. & Zhang, Y. Lewis pairs polymerization of polar vinyl monomers. Sci. Bull. 64, 1830–1840 (2019).
Wang, Q. et al. Living polymerization of conjugated polar alkenes catalyzed by N-heterocyclic olefin-based frustrated Lewis pairs. ACS Catal. 8, 3571–3578 (2018).
Wang, H., Wang, Q., He, J. & Zhang, Y. Living polymerization of acrylamides catalysed by N-heterocyclic olefin-based Lewis pairs. Polym. Chem. 10, 3597–3603 (2019).
Zhang, P., Zhou, H. & Lu, X.-B. Living and chemoselective (co)polymerization of polar divinyl monomers mediated by bulky Lewis pairs. Macromolecules 52, 4520–4525 (2019).
Bai, Y., He, J. & Zhang, Y. Ultra-high-molecular-weight polymers produced by the immortal phosphine-based catalyst system. Angew. Chem. Int. Ed. 57, 17230–17234 (2018).
Wang, Q., Zhao, W., He, J., Zhang, Y. & Chen, E. Y.-X. Living ring-opening polymerization of lactones by N-heterocyclic olefin/Al(C6F5)3 Lewis pairs: structures of intermediates, kinetics, and mechanism. Macromolecules 50, 123–136 (2017).
Zhang, H., Nie, Y., Zhi, X., Du, H. & Yang, J. Controlled ring-opening polymerization of α-amino acid N-carboxy-anhydride by frustrated amine/borane Lewis pairs. Chem. Commun. 53, 5155–5158 (2017).
Naumann, S., Scholten, P. B., Wilson, J. A. & Dove, A. P. Dual catalysis for selective ring-opening polymerization of lactones: evolution toward simplicity. J. Am. Chem. Soc. 137, 14439–14445 (2015).
Ji, H.-Y., Wang, B., Pan, L. & Li, Y.-S. Lewis pairs for ring-opening alternating copolymerization of cyclic anhydrides and epoxides. Green Chem. 20, 641–648 (2018).
Yang, J.-L., Wu, H.-L., Li, Y., Zhang, X.-H. & Darensbourg, D. J. Perfectly alternating and regioselective copolymerization of carbonyl sulfide and epoxides by metal-free Lewis pairs. Angew. Chem. Int. Ed. 56, 5774–5779 (2017).
Zhang, D., Boopathi, S. K., Hadjichristidis, N., Gnanou, Y. & Feng, X. Metal-free alternating copolymerization of CO2 with epoxides: fulfilling “green” synthesis and activity. J. Am. Chem. Soc. 138, 11117–11120 (2016).
Zhang, J., Wang, L., Liu, S., Kang, X. & Li, Z. A Lewis pair as organocatalyst for one-pot synthesis of block copolymers from a mixture of epoxide, anhydride, and CO2. Macromolecules 54, 763–772 (2021).
Wang, Y. et al. Highly selective and productive synthesis of a carbon dioxide-based copolymer upon zwitterionic growth. Macromolecules 54, 2178–2186 (2021).
Liu, S. et al. Biased Lewis pairs: a general catalytic approach to ether-ester block copolymers with unlimited ordering of sequences. Angew. Chem. Int. Ed. 58, 15478–15487 (2019).
Bai, Y., Wang, H., He, J. & Zhang, Y. Rapid and scalable access to sequence‐controlled DHDM multiblock copolymers by FLP polymerization. Angew. Chem. Int. Ed. 59, 11613–11619 (2020).
McGraw, M. L., Clarke, R. W. & Chen, E. Y.-X. Compounded sequence control in polymerization of one-pot mixtures of highly reactive acrylates by differentiating Lewis pairs. J. Am. Chem. Soc. 142, 5969–5973 (2020).
McGraw, M. L., Clarke, R. W. & Chen, E. Y.-X. Synchronous control of chain length/sequence/topology for precision synthesis of cyclic block copolymers from monomer mixtures. J. Am. Chem. Soc. 143, 3318–3322 (2021).
Hosoi, Y., Takasu, A., Matsuoka, S. & Hayashi, M. N-Heterocyclic carbene initiated anionic polymerization of (E,E)-methyl sorbate and subsequent ring-closing to cyclic poly(alkyl sorbate). J. Am. Chem. Soc. 139, 15005–15012 (2017).
Oga, Y., Hosoi, Y. & Takasu, A. Synthesis of cyclic poly(methyl methacrylate) via N-heterocyclic carbene (NHC) initiated-anionic polymerization and subsequent ring-closing without need of highly dilute conditions. Polymer 186, 122019 (2020).
Wang, B., Pan, L., Ma, Z. & Li, Y. Ring-opening polymerization with Lewis pairs and subsequent nucleophilic substitution: a promising strategy to well-defined polyethylene-like polyesters without transesterification. Macromolecules 51, 836–845 (2018).
Wang, L.-Y. et al. Intramolecularly cooperative catalysis for copolymerization of cyclic thioanhydrides and epoxides: a dual activation strategy to well-defined polythioesters. ACS Catal. 10, 6635–6644 (2020).
Yang, G.-W., Zhang, Y.-Y., Xie, R. & Wu, G.-P. Scalable bifunctional organoboron catalysts for copolymerization of CO2 and epoxides with unprecedented efficiency. J. Am. Chem. Soc. 142, 12245–12255 (2020).
Yang, G.-W., Zhang, Y.-Y., Xie, R. & Wu, G.-P. High-activity organocatalysts for polyether synthesis via intramolecular ammonium cation assisted SN2 ring-opening polymerization. Angew. Chem. Int. Ed. 59, 16910–16917 (2020).
Yang, G.-W. et al. Pinwheel-shaped tetranuclear organoboron catalysts for perfectly alternating copolymerization of CO2 and epichlorohydrin. J. Am. Chem. Soc. 143, 3455–3465 (2021).
Wang, L. et al. Cooperative carbon monoxide to formyl reduction at a trifunctional PBB frustrated Lewis pair. Chem. Commun. 53, 5499–5502 (2017).
Wang, L. et al. Formation of macrocyclic ring systems by carbonylation of trifunctional P/B/B frustrated Lewis pairs. Chem. Sci. 9, 1544–1550 (2018).
Zhang, Y. et al. Lewis pair polymerization by classical and frustrated Lewis pairs: acid, base and monomer scope and polymerization mechanism. Dalton Trans. 41, 9119–9134 (2012).
Gowda, R. R. & Chen, E. Y.-X. Sustainable Polymers from Biomass-Derived α-Methylene-γ-Butyrolactones. in Mark, H. F. Ed. Encyclopedia of Polymer Science and Technology, 4th Ed, Vol. 8, pp. 235–271; Wiley, Hoboken, 2014.
Agarwal, S., Jin, Q. & Maji, S. Biobased polymers from plant-derived Tulipalin A. ACS Symp. Ser. 1105, 197–212 (2012).
Bai, Y., Wang, H., He, J., Zhang, Y. & Chen, E. Y.-X. Dual-initiating and living frustrated Lewis pairs: expeditious synthesis of biobased thermoplastic elastomers. Nat. Commun. 12, 4874 (2021).
Gilsdorf, R. A., Nicki, M. A. & Chen, E. Y.-X. High chemical recyclability of vinyl lactone acrylic bioplastics. Polym. Chem. 11, 4942–4950 (2020).
He, J. et al. Chain propagation and termination mechanisms for polymerization of conjugated polar alkenes by [Al]-based frustrated Lewis pairs. Macromolecules 47, 7765–7774 (2014).
Xu, P. & Xu, X. Homoleptic rare-earth aryloxide based Lewis pairs for polymerization of conjugated polar alkenes. ACS Catal. 8, 198–202 (2018).
Kaminsky, W. & Franck, J. Monomer recovery by pyrolysis of poly(methyl methacrylate) (PMMA). J. Anal. Appl. Pyrolysis 19, 311–318 (1991).
Kuprat, M., Lehmann, M., Schulz, A. & Villinger, A. Synthesis of pentafluorophenyl silver by means of Lewis acid catalysis: structure of silver solvent complexes. Organometallics 29, 1421–1427 (2010).
Parks, D. J., Piers, W. E. & Yap, G. P. A. Synthesis, properties, and hydroboration activity of the highly electrophilic borane bis(pentafluorophenyl)borane, HB(C6F5)2. Organometallics 17, 5492–5503 (1998).
Sudhakar, G., Satish, K. & Raghavaiah, J. Total synthesis and absolute configuration of curvularides A–E. J. Org. Chem. 77, 10010–10020 (2012).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 22071077 and 21871107) to Y.Z. and the US National Science Foundation (NSF-1904962) to E.Y.-X.C. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.Z. and Y.S. conceived the idea and designed the experiments. Y.S. and R.A.G performed the experiments and characterizations. Y.S., J.H., Y.Z. and E.Y.-X.C. analysed the data, wrote parts of the manuscript and provided input. Y.Z. directed the project.
Corresponding author
Ethics declarations
Competing interests
Y.Z., Y.S. and J.H. are named inventors on a Chinese patent application (number 202111184369.9) submitted by Jilin University that covers the preparation and characterization of cyclic PMMBL. The other authors (R.A.G. and E. Y.-X.C.) declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Kai Guo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods 1–5, Figs. 1–46 and Tables 1 and 2.
Supplementary Video 1
Mass spectra collected from the degradation of l-PMMBL scanned from 30 to 623 °C at 10 °C min−1, m/z from 1 to 130 amu, showing evolution of ion reflux. Source data for Supplementary Fig. 37.
Supplementary Video 2
Mass spectra collected from the degradation of c-PMMBL scanned from 30 °C to 623 °C at 10 °C min−1, m/z from 1 to 130 amu, showing evolution of ion reflux. Source data for Supplementary Fig. 40.
Supplementary Video 3
Mass spectra collected from the degradation of l-PMMBL scanned during isothermal treatment at 300 °C for 60 min, m/z from 1 to 130 amu, showing evolution of ion reflux. Snapshot shown in Supplementary Fig. 42.
Supplementary Video 4
Mass spectra collected from the degradation of c-PMMBL scanned during isothermal treatment at 300 °C for 60 min, m/z from 1 to 130 amu, showing evolution of ion reflux. Snapshot shown in Supplementary Fig. 44.
Supplementary Data 1
Crystal data and structure refinement, and bond lengths and angles for compound 1A; CCDC reference 2111456.
Supplementary Data 2
Crystallographic data for compound 1A; CCDC reference 2111456.
Supplementary Data 3
Structure factor file for compound 1A; CCDC reference 2111456.
Supplementary Data 4
Crystal data and structure refinement, and bond lengths and angles for compound 2; CCDC reference 2111528.
Supplementary Data 5
Crystallographic data for compound 2; CCDC reference 2111528.
Supplementary Data 6
Structure factor file for compound 2; CCDC reference 2111528.
Supplementary Data 7
Supplementary statistical source data for the polymerization kinetics of Supplementary Fig. 24.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Y., He, J., Zhang, Y. et al. Recyclable cyclic bio-based acrylic polymer via pairwise monomer enchainment by a trifunctional Lewis pair. Nat. Chem. 15, 366–376 (2023). https://doi.org/10.1038/s41557-022-01097-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01097-7
This article is cited by
-
Chemoselective and Living/Controlled Polymerization of Alkenyl Methacrylates by the Phosphonium Ylide/Organoaluminum Lewis Pairs
Chinese Journal of Polymer Science (2024)
-
A recyclable polyester library from reversible alternating copolymerization of aldehyde and cyclic anhydride
Nature Communications (2023)
-
Research progress in the living/controlled polymerization of (meth)acrylate monomers by Lewis pair
Science China Chemistry (2023)