Abstract
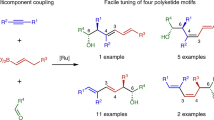
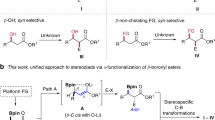
Polyketide natural products often contain common repeat motifs, for example, propionate, acetate and deoxypropionate, and so can be synthesized by iterative processes. We report here a highly efficient iterative strategy for the synthesis of polyacetates based on boronic ester homologation that does not require functional group manipulation between iterations. This process involves sequential asymmetric diboration of a terminal alkene, forming a 1,2-bis(boronic ester), followed by regio- and stereoselective homologation of the primary boronic ester with a butenyl metallated carbenoid to generate a 1,3-bis(boronic ester). Each transformation independently controls the stereochemical configuration, making the process highly versatile, and the sequence can be iterated prior to stereospecific oxidation of the 1,3-polyboronic ester to yield the 1,3-polyol. This methodology has been applied to a 14-step synthesis of the oxopolyene macrolide bahamaolide A, and the versatility of the 1,3-polyboronic esters has been demonstrated in various stereospecific transformations, leading to polyalkenes, -alkynes, -ketones and -aromatics with full stereocontrol.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this work are provided in the Supplementary Information. The crystallographic data for the structure of boronic ester 25 reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2150709. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661 (2016).
Staunton, J. & Weissman, K. J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 18, 380–416 (2001).
Helfrich, E. J. N. & Piel, J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 33, 231–316 (2016).
Hwang, S., Lee, N., Cho, S., Palsson, B. & Cho, B.-K. Repurposing modular polyketide synthases and non-ribosomal peptide synthetases for novel chemical biosynthesis. Front. Mol. Biosci. 87 (2020).
Ma, L. et al. Assembly Line and Post-PKS Modifications in the Biosynthesis of Marine Polyketide Natural Products. in Liu,H.-W. & Begley, T. P. (eds.) Comprehensive Natural Products III 139–197 (Elsevier, 2020).
Zheng, K., Xie, C. & Hong, R. Bioinspired iterative synthesis of polyketides. Front. Chem. 3, 32 (2015).
Lehmann, J. W., Blair, D. J. & Burke, M. D. Towards the generalized iterative synthesis of small molecules. Nat. Rev. Chem. 2, 115 (2018).
ter Horst, B., Feringa, B. L. & Minnaard, A. J. Iterative strategies for the synthesis of deoxypropionates. Chem. Commun. 46, 2535–2547 (2010).
García-Fortanet, J., Murga, J., Carda, M. & Marco, J. A. On the structure of passifloricin A: asymmetric synthesis of the δ-lactones of (2Z,5S,7R,9S,11S)- and (2Z,5R,7R,9S,11S)-tetrahydroxyhexacos-2-enoic acid. Org. Lett. 5, 1447–1449 (2003).
Zhang, Y., Arpin, C. C., Cullen, A. J., Mitton-Fry, M. J. & Sammakia, T. Total synthesis of dermostatin A. J. Org. Chem. 76, 7641–7653 (2011).
Kim, I. S., Ngai, M.-Y. & Krische, M. J. Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level using allyl acetate as an allyl metal surrogate. J. Am. Chem. Soc. 130, 6340–6341 (2008).
Han, S. B., Hassan, A., Kim, I. S. & Krische, M. J. Total synthesis of (+)-roxaticin via C–C bond forming transfer hydrogenation: a departure from stoichiometric chiral reagents, auxiliaries, and premetalated nucleophiles in polyketide construction. J. Am. Chem. Soc. 132, 15559–15561 (2010).
Feng, J., Kasun, Z. A. & Krische, M. J. Enantioselective alcohol C−H functionalization for polyketide construction: unlocking redox-economy and site-selectivity for ideal chemical synthesis. J. Am. Chem. Soc. 138, 5467–5478 (2016).
Romea, P. & Urpí, F. in Modern Methods in Stereoselective Aldol Reactions (ed. Mahrwald, R.) 1–81 (Wiley, 2013).
Carreira, E. M., Singer, R. A. & Lee, W. Catalytic, enantioselective aldol additions with methyl and ethyl acetate O-silyl enolates: a chiral tridentate chelate as a ligand for titanium(IV). J. Am. Chem. Soc. 116, 8837–8838 (1994).
Kim, Y., Singer, R. A. & Carreira, E. M. Total synthesis of macrolactin A with versatile catalytic, enantioselective dienolate aldol addition reactions. Angew. Chem. Int. Ed. 37, 1261–1263 (1998).
Evans, D. A. & Connell, B. T. Synthesis of the antifungal macrolide antibiotic (+)-roxaticin. J. Am. Chem. Soc. 125, 10899–10905 (2003).
Dias, L. C., Kuroishi, P. K. & De Lucca, E. C. The total synthesis of (−)-cryptocaryol A. Org. Biomol. Chem. 13, 3575–3584 (2015).
Rychnovsky, S. D. & Hoye, R. C. Convergent synthesis of the polyene macrolide (−)-roxaticin. J. Am. Chem. Soc. 116, 1753–1765 (1994).
Richardson, T. I. & Rychnovsky, S. D. Total synthesis of filipin III. J. Am. Chem. Soc. 119, 12360–12361 (1997).
Deng, Y. & Smith, A. B. III Evolution of anion relay chemistry: construction of architecturally complex natural products. Acc. Chem. Res. 53, 988–1000 (2020).
Leonori, D. & Aggarwal, V. K. Lithiation−borylation methodology and its application in synthesis. Acc. Chem. Res. 47, 3174–3183 (2014).
Burns, M. et al. Assembly-line synthesis of organic molecules with tailored shapes. Nature 513, 183–188 (2014).
Balieu, S. et al. Toward ideality: the synthesis of (+)-kalkitoxin and (+)-hydroxyphthioceranic acid by assembly-line synthesis. J. Am. Chem. Soc. 137, 4398–4403 (2015).
Bootwicha, T., Feilner, J. M., Myers, E. L. & Aggarwal, V. K. Iterative assembly line synthesis of polypropionates with full stereocontrol. Nat. Chem. 9, 896–902 (2017).
Wu, J. et al. Synergy of synthesis, computation and NMR reveals correct baulamycin structures. Nature 547, 436–440 (2017).
Pradeilles, J. A. et al. Odd–even alternations in helical propensity of a homologous series of hydrocarbons. Nat. Chem. 12, 475–480 (2020).
Yeung, K., Mykura, R. C. & Aggarwal, V. K. Lithiation–borylation methodology in the total synthesis of natural products. Nat. Synth. 1, 117–126 (2022).
Coombs, J. R., Haeffner, F., Kliman, L. T. & Morken, J. P. Scope and mechanism of the Pt-catalyzed enantioselective diboration of monosubstituted alkenes. J. Am. Chem. Soc. 135, 11222–11231 (2013).
Fawcett, A. et al. Regio- and stereoselective homologation of 1,2-bis(boronic esters): stereocontrolled synthesis of 1,3-diols and Sch 725674. Angew. Chem. Int. Ed. 55, 14663–14667 (2016).
Casoni, G. et al. Sulfinyl benzoates as precursors to Li and Mg carbenoids for the stereoselective iterative homologation of boronic esters. J. Am. Chem. Soc. 139, 11877–11886 (2017).
Kim, D. G. et al. Bahamaolides A and B, antifungal polyene polyol macrolides from the marine actinomycete Streptomyces sp. J. Nat. Prod. 75, 959–967 (2012).
Lee, S.-H. et al. Bahamaolide A from the marine-derived Streptomyces sp. CNQ343 inhibits isocitrate lyase in Candida albicans. Bioorg. Med. Chem. Lett. 24, 4291–4293 (2014).
Mori, Y., Asai, M., Kawade, J. & Furukawa, H. Total synthesis of the polyene macrolide antibiotic roxaticin. II. Total synthesis of roxaticin. Tetrahedron 51, 5315–5330 (1995).
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009).
Sandford, C. & Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 53, 5481–5494 (2017).
Renaudet, O. & Roy, R. Multivalent scaffolds in glycoscience: an overview. Chem. Soc. Rev. 42, 4515–4517 (2013).
Guan, X., Chen, F., Fang, Q. & Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 49, 1357–1384 (2020).
Stadtmueller, M. N. et al. Evaluation of multivalent sialylated polyglycerols for resistance induction in and broad antiviral activity against influenza A viruses. J. Med. Chem. 64, 12774–12789 (2021).
Bhatia, S., Dimde, M. & Haag, R. Multivalent glycoconjugates as vaccines and potential drug candidates. MedChemComm 5, 862–878 (2014).
Mammen, M., Choi, S.-K. & Whitesides, G. M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 37, 2754–2794 (1998).
Bhatia, S. et al. Linear polysialoside outperforms dendritic analogs for inhibition of influenza virus infection in vitro and in vivo. Biomaterials 138, 22–34 (2017).
Vonnemann, J. et al. Size dependence of steric shielding and multivalency effects for globular binding inhibitors. J. Am. Chem. Soc. 137, 2572–2579 (2015).
Zweifel, G., Arzoumanian, H. & Whitney, C. C. A convenient stereoselective synthesis of substituted alkenes via hydroboration–iodination of alkynes. J. Am. Chem. Soc. 89, 3652–3653 (1967).
Armstrong, R. J. & Aggarwal, V. K. 50 years of Zweifel olefination: a transition-metal-free coupling. Synthesis (Stuttg.) 49, 3323–3336 (2017).
Wang, Y., Noble, A., Myers, L. & Aggarwal, V. K. Enantiospecific alkynylation of alkylboronic esters. Angew. Chem. Int. Ed. 55, 4270–4274 (2016).
Mukherjee, C., Mäkinen, K., Savolainen, J. & Leino, R. Chemistry and biology of oligovalent β-(1→2)-linked oligomannosides: new insights into carbohydrate-based adjuvants in immunotherapy. Chem. Eur. J. 19, 7961–7974 (2013).
Geng, Z., Shin, J. J., Xi, Y. & Hawker, C. J. Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci. 59, 963–1042 (2021).
Bonet, A., Odachowski, M., Leonori, D., Essafi, S. & Aggarwal, V. K. Enantiospecific sp2–sp3 coupling of secondary and tertiary boronic esters. Nat. Chem. 6, 584–589 (2014).
Coates, G. W. & Grubbs, R. Quantitative ring-closing metathesis of polyolefins. J. Am. Chem. Soc. 118, 229–230 (1996).
Acknowledgements
We thank H2020 ERC (no. 670668, V.K.A.) for financial support. H.-H.L. and T.B thank the Marie Skłodowska-Curie Fellowship programme for support (Horizon 2020, no. 746045 (H.-H.L.) and no. 626828 (T.B.)). We thank N. Pridmore and H. Sparkes for X-ray analysis, D. J. Blair for useful discussions and C. P. Butts for assistance with NMR analysis.
Author information
Authors and Affiliations
Contributions
S.G.A., J.M.B. and H.-H.L. contributed equally to this work. S.G.A., J.M.B., H.-H.L, A.F. and T.B. conducted the experimental work and analysed the data. All authors contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures and characterization for all compounds synthesized in this work, Supplementary discussion, Supplementary Figs. 1–4, Supplementary Schemes 1–13 and Supplementary Tables 1–10.
Supplementary Data
Crystallographic data for compound 25: CCDC 2150709.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aiken, S.G., Bateman, J.M., Liao, HH. et al. Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A. Nat. Chem. 15, 248–256 (2023). https://doi.org/10.1038/s41557-022-01087-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01087-9