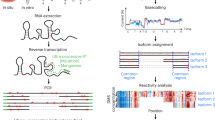
Abstract
Identifying RNA transcript isoforms requires intricate protocols that suffer from various enzymatic biases. Here we design three-dimensional molecular constructs that enable identification of transcript isoforms at the single-molecule level using solid-state nanopore microscopy. We refold target RNA into RNA identifiers with designed sets of complementary DNA strands. Each reshaped molecule carries a unique sequence of structural (pseudo)colours. Structural colours consist of DNA structures, protein labels, native RNA structures or a combination of all three. The sequence of structural colours of RNA identifiers enables simultaneous identification and relative quantification of multiple RNA targets without prior amplification. Our Amplification-free RNA TargEt Multiplex Isoform Sensing (ARTEMIS) method reveals structural arrangements in native transcripts in agreement with published variants. ARTEMIS discriminates circular and linear transcript isoforms in a one-step, enzyme-free reaction in a complex human transcriptome using single-molecule read-out.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the findings of this study are available in the main text and the Supplementary Information. Additional raw data are available at https://doi.org/10.17863/CAM.87123. Source data are provided with this paper.
References
Ozsolak, F. & Milos, P. M. RNA sequencing: advances, challenges and opportunities. Nat. Rev. Genet. 12, 87–98 (2011).
Jeck, W. R. & Sharpless, N. E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 32, 453–461 (2014).
Harvey, S. E. & Cheng, C. Methods for characterization of alternative RNA splicing. Methods Mol. Biol. 1402, 229–241 (2016).
Heid, C. A., Stevens, J., Livak, K. J. & Williams, P. M. Real time quantitative PCR. Genome Res. 6, 986–994 (1996).
Stark, R., Grzelak, M. & Hadfield, J. RNA sequencing: the teenage years. Nat. Rev. Genet. 20, 631–656 (2019).
Gootenberg, J. S. et al. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a and Csm6. Science 360, 439–444 (2018).
Mamanova, L. et al. FRT-seq: amplification-free, strand-specific transcriptome sequencing. Nat. Methods 7, 130–132 (2010).
McGettigan, P. A. Transcriptomics in the RNA-seq era. Curr. Opin. Chem. Biol. 17, 4–11 (2013).
Meyer, C. A. & Liu, X. S. Identifying and mitigating bias in next-generation sequencing methods for chromatin biology. Nat. Rev. Genet. 15, 709–721 (2014).
Garalde, D. R. et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods 15, 201–206 (2018).
Conn, V. & Conn, S. J. SplintQuant: a method for accurately quantifying circular RNA transcript abundance without reverse transcription bias. RNA 25, 1202–1210 (2019).
Workman, R. E. et al. Nanopore native RNA sequencing of a human poly(A) transcriptome. Nat. Methods 16, 1297–1305 (2019).
Guo, Y., Li, C. I., Ye, F. & Shyr, Y. Evaluation of read count based RNAseq analysis methods. BMC Genomics 14, S2 (2013).
Soneson, C. et al. A comprehensive examination of nanopore native RNA sequencing for characterization of complex transcriptomes. Nat. Commun. 10, 3359 (2019).
Li, J., Jiang, H. & Wong, W. H. Modeling non-uniformity in short-read rates in RNA-Seq data. Genome Biol. 11, R50 (2010).
Devenson, I. W. et al. Universal alternative splicing of noncoding exons. Cell Syst. 6, 245–255.e5 (2018).
Lagarde, J. et al. High-throughput annotation of full-length long noncoding RNAs with capture long-read sequencing. Nat. Genet. 49, 1731–1740 (2017).
Rothemund, P. W. K. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302 (2006).
Howarth, M. et al. A monovalent streptavidin with a single femtomolar biotin binding site. Nat. Methods 3, 267–273 (2006).
Li, J., Gershow, M., Stein, D., Brandin, E. & Golovchenko, J. A. DNA molecules and configurations in a solid-state nanopore microscope. Nat. Mater. 2, 611–615 (2003).
Deamer, D., Akeson, M. & Branton, D. Three decades of nanopore sequencing. Nat. Biotechnol. 34, 518–524 (2016).
Dekker, C. Solid-state nanopores. Nat. Nanotechnol. 2, 209–215 (2007).
Plesa, C., Van Loo, N., Ketterer, P., Dietz, H. & Dekker, C. Velocity of DNA during translocation through a solid-state nanopore. Nano Lett. 15, 732–737 (2015).
Roberts, R. W. & Crothers, D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science 258, 1463–1466 (1992).
Müller, S. & Appel, B. In vitro circularization of RNA. RNA Biology 14, 1018–1027 (2017).
Amarasinghe, S. L. et al. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 21, 30 (2020).
Popenda, M. et al. Automated 3D structure composition for large RNAs. Nucleic Acids Res. 40, e112 (2012).
Uhlen, M. et al. A pathology atlas of the human cancer transcriptome. Science 357, eaan2507 (2017).
Howe, K. L. et al. Ensembl 2021. Nucleic Acids Res. 49, D884–D891 (2021).
Kuchipudi, S. V. et al. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol. J. 9, 230 (2012).
Bell, N. A. W., Muthukumar, M. & Keyser, U. F. Translocation frequency of double-stranded DNA through a solid-state nanopore. Phys. Rev. E 93, 022401 (2016).
Eyras, E., Caccamo, M., Curwen, V. & Clamp, M. ESTGenes: alternative splicing from ESTs in Ensembl. Genome Res. 14, 976–987 (2004).
Steinhauer, C., Jungmann, R., Sobey, T. L., Simmel, F. C. & Tinnefeld, P. DNA origami as a nanoscopic ruler for superresolution microscopy. Angew. Chem. Int. Ed. 48, 8870–8873 (2009).
Huang, G., Voet, A. & Maglia, G. FraC nanopores with adjustable diameter identify the mass of opposite-charge peptides with 44 dalton resolution. Nat. Commun. 10, 835 (2019).
Morillon, A. & Gautheret, D. Bridging the gap between reference and real transcriptomes. Genome Biol. 20, 112 (2019).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018).
Zhang, X. et al. Comparative analysis of droplet-based ultra-high-throughput single-cell RNA-seq systems. Mol. Cell 73, 130–142.e5 (2019).
Han, D. et al. Single-stranded DNA and RNA origami. Science 358, eaao2648 (2017).
Liu, D. et al. Branched kissing loops for the construction of diverse RNA homooligomeric nanostructures. Nat. Chem. 12, 249–259 (2020).
Geary, C., Grossi, G., McRae, E. K. S., Rothemund, P. W. K. & Andersen, E. S. RNA origami design tools enable cotranscriptional folding of kilobase-sized nanoscaffolds. Nat. Chem. 13, 549–558 (2021).
Acknowledgements
We thank J. Zhu and M. Fletcher for the critical reading of the manuscript and useful suggestions. We thank the Howarth Lab from the University of Oxford for the monovalent streptavidin. U.F.K. acknowledges funding from a European Research Council Consolidator grant (DesignerPores no. 647144) and European Research Council Proof-Of-Concept grant (PoreDetect no. 899538). F.B. acknowledges funding from George and Lilian Schiff Foundation Studentship, the Winton Programme for the Physics of Sustainability Ph.D. Scholarship and St John’s College Benefactors’ Scholarship.
Author information
Authors and Affiliations
Contributions
F.B. conceived the idea. F.B. and U.F.K. designed the study. F.B. performed the experiments and analysed the data. F.B. and U.F.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
F.B. and U.F.K. are inventors for the ARTEMIS method (United Kingdom patent application no. 2113935.7, in process) submitted by Cambridge Enterprise on the behalf of the University of Cambridge. U.F.K. is a cofounder of Cambridge Nucleomics.
Peer review
Peer review information
Nature Chemistry thanks Sergii Pud, Adam Hall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Tables 1–16, Materials and Methods, and text.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data and RNA structure prediction files.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bošković, F., Keyser, U.F. Nanopore microscope identifies RNA isoforms with structural colours. Nat. Chem. 14, 1258–1264 (2022). https://doi.org/10.1038/s41557-022-01037-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01037-5
This article is cited by
-
Nanopore DNA sequencing technologies and their applications towards single-molecule proteomics
Nature Chemistry (2024)
-
Single-molecule RNA sizing enables quantitative analysis of alternative transcription termination
Nature Communications (2024)
-
Electrochemical Analysis of Single Glucose Oxidase with a Nanopipette
Chemical Research in Chinese Universities (2024)