Abstract
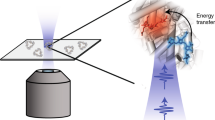
The phycobilisome is an oligomeric chromoprotein complex that serves as the principal mid-visible light-harvesting system in cyanobacteria. Here we report the observation of excitation-energy-transfer pathways involving delocalized optical excitations of the bilin (linear tetrapyrrole) chromophores in intact phycobilisomes isolated from Fremyella diplosiphon. By using broadband multidimensional electronic spectroscopy with 6.7-fs laser pulses, we are able to follow the progress of excitation energy from the phycoerythrin disks at the ends of the phycobilisome’s rods to the C-phycocyanin disks along their length in <600 fs. Oscillation maps show that coherent wavepacket motions prominently involving the hydrogen out-of-plane vibrations of the bilins mediate non-adiabatic relaxation of a manifold of vibronic exciton states. However, the charge-transfer character of the bilins in the allophycocyanin-containing segments localizes the excitations in the core of the phycobilisome, yielding a kinetic bottleneck that enables photoregulatory mechanisms to operate efficiently on the >10-ps timescale.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available at https://doi.org/10.5281/zenodo.6743715 (ref. 75). Source data are provided with this paper.
Code availability
The MATLAB and Julia scripts employed for analysis of data during the current study are available at https://doi.org/10.5281/zenodo.6743715 (ref. 75).
References
Gantt, E. Phycobilisomes: light-harvesting pigment complexes. Bioscience 25, 781–788 (1975).
Glazer, A. N. Light harvesting by phycobilisomes. Annu. Rev. Biophys. Biophys. Chem. 14, 47–77 (1985).
Adir, N. Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth. Res. 85, 15–32 (2005).
David, L., Marx, A. & Adir, N. High-resolution crystal structures of trimeric and rod phycocyanin. J. Mol. Biol. 405, 201–213 (2011).
Liu, H. et al. Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science 342, 1104–1107 (2013).
van Stokkum, I. H. M. et al. A functional compartmental model of the Synechocystis PCC 6803 phycobilisome. Photosynth. Res. 135, 87–102 (2018).
Tian, L. et al. Picosecond kinetics of light harvesting and photoprotective quenching in wild-type and mutant phycobilisomes isolated from the cyanobacterium Synechocystis PCC 6803. Biophys. J. 102, 1692–1700 (2012).
Sauer, K. & Scheer, H. Excitation transfer in C-phycocyanin. Förster transfer rate and exciton calculations based on new crystal structure data for C-phycocyanins from Agmenellum quadruplicatum and Mastigocladus laminosus. Biochim. Biophys. Acta 936, 157–170 (1988).
Debreczeny, M. P., Sauer, K., Zhou, J. & Bryant, D. A. Comparison of calculated and experimentally resolved rate constants for excitation energy transfer in C-phycocyanin. 2. Trimers. J. Phys. Chem. 99, 8420–8431 (1995).
Beljonne, D., Curutchet, C., Scholes, G. D. & Silbey, R. J. Beyond Förster resonance energy transfer in biological and nanoscale systems. J. Phys. Chem. B 113, 6583–6599 (2009).
Riter, R. E., Edington, M. D. & Beck, W. F. Isolated-chromophore and exciton-state photophysics in C-phycocyanin trimers. J. Phys. Chem. B 101, 2366–2371 (1997).
Homoelle, B. J., Edington, M. D., Diffey, W. M. & Beck, W. F. Stimulated photon-echo and transient-grating studies of protein-matrix solvation dynamics and interexciton-state radiationless decay in α phycocyanin and allophycocyanin. J. Phys. Chem. B 102, 3044–3052 (1998).
Womick, J. M. & Moran, A. M. Exciton coherence and energy transport in the light-harvesting dimers of allophycocyanin. J. Phys. Chem. B 113, 15747–15759 (2009).
Womick, J. M. & Moran, A. M. Nature of excited states and relaxation mechanisms in C-phycocyanin. J. Phys. Chem. B 113, 15771–15782 (2009).
Womick, J. M., Liu, H. & Moran, A. M. Exciton delocalization and energy transport mechanisms in R-phycoerythrin. J. Phys. Chem. A 115, 2471–2482 (2011).
Theiss, C. et al. Excitation energy transfer in intact cells and in the phycobiliprotein antennae of the chlorophyll d containing cyanobacterium Acaryochloris marina. J. Plant Physiol. 168, 1473–1487 (2011).
Nganou, C., David, L., Adir, N. & Mkandawire, M. Linker proteins enable ultrafast excitation energy transfer in the phycobilisome antenna system of Thermosynechococcus vulcanus. Photochem. Photobiol. Sci. 15, 31–44 (2016).
Fălămaș, A., Porav, S. A. & Tosa, V. Investigations of the energy transfer in the phycobilisome antenna of Arthrospira platensis using femtosecond spectroscopy. NATO Adv. Sci. Inst. Ser. E Appl. Sci. 10, 4045 (2020).
Zheng, L. et al. Structural insight into the mechanism of energy transfer in cyanobacterial phycobilisomes. Nat. Commun. 12, 5497 (2021).
Zhang, J. et al. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 551, 57–63 (2017).
Ma, J. et al. Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature 579, 146–151 (2020).
Jonas, D. M. Two-dimensional femtosecond spectroscopy. Annu. Rev. Phys. Chem. 54, 425–463 (2003).
Li, H., Bristow, A. D., Siemens, M. E., Moody, G. & Cundiff, S. T. Unraveling quantum pathways using optical 3D Fourier-transform spectroscopy. Nat. Commun. 4, 1390 (2013).
van Stokkum, I. H. M., Larsen, D. S. & van Grondelle, R. Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta 1657, 82–104 (2004).
Tian, L. et al. Site, rate and mechanism of photoprotective quenching in cyanobacteria. J. Am. Chem. Soc. 133, 18304–18311 (2011).
Krüger, T. P. J., van Grondelle, R. & Gwizdala, M. The role of far-red spectral states in the energy regulation of phycobilisomes. Biochim. Biophys. Acta Bioenerg. 1860, 341–349 (2019).
Wahadoszamen, M., Krüger, T. P. J., Ara, A. M., van Grondelle, R. & Gwizdala, M. Charge transfer states in phycobilisomes. Biochim. Biophys. Acta Bioenerg. 1861, 148187 (2020).
Edington, M. D., Riter, R. E. & Beck, W. F. Interexciton-state relaxation and exciton localization in allophycocyanin trimers. J. Phys. Chem. 100, 14206–14217 (1996).
Cheng, Y.-C. & Fleming, G. R. Coherence quantum beats in two-dimensional electronic spectroscopy. J. Phys. Chem. A 112, 4254–4260 (2008).
Ginsberg, N. S., Cheng, Y.-C. & Fleming, G. R. Two-dimensional electronic spectroscopy of molecular aggregates. Acc. Chem. Res. 42, 1352–1363 (2009).
Collini, E. et al. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 463, 644–647 (2010).
Butkus, V., Zigmantas, D., Valkunas, L. & Abramavicius, D. Vibrational vs. electronic coherences in 2D spectrum of molecular systems. Chem. Phys. Lett. 545, 40–43 (2012).
Duan, H.-G. et al. Nature does not rely on long-lived electronic quantum coherence for photosynthetic energy transfer. Proc. Natl Acad. Sci. USA 114, 8493–8498 (2017).
Tiwari, V., Peters, W. K. & Jonas, D. M. Electronic resonance with anticorrelated pigment vibrations drives photosynthetic energy transfer outside the adiabatic framework. Proc. Natl Acad. Sci. USA 110, 1203–1208 (2013).
Butkus, V. et al. Discrimination of diverse coherences allows identification of electronic transitions of a molecular nanoring. J. Phys. Chem. Lett. 8, 2344–2349 (2017).
Kneip, C., Hildebrandt, P., Németh, K., Mark, F. & Schaffner, K. Interpretation of the resonance Raman spectra of linear tetrapyrroles based on DFT calculations. Chem. Phys. Lett. 311, 479–484 (1999).
Kneip, C. et al. Protonation state and structural changes of the tetrapyrrole chromophore during the Pr → Pfr phototransformation of phytochrome: a resonance Raman spectroscopic study. Biochemistry 38, 15185–15192 (1999).
Andel, F. III et al. Probing the photoreaction mechanism of phytochrome through analysis of resonance Raman vibrational spectra of recombinant analogues. Biochemistry 39, 2667–2676 (2000).
Dasgupta, J., Frontiera, R. R., Taylor, K. C., Lagarias, J. C. & Mathies, R. A. Ultrafast excited-state isomerization in phytochrome revealed by femtosecond stimulated Raman spectroscopy. Proc. Natl Acad. Sci. USA 106, 1784–1789 (2009).
Osoegawa, S. et al. Identification of the deprotonated pyrrole nitrogen of the bilin-based photoreceptor by Raman spectroscopy with an advanced computational analysis. J. Phys. Chem. B 123, 3242–3247 (2019).
Pollard, W. T. & Mathies, R. A. Analysis of femtosecond dynamic absorption spectra of nonstationary states. Annu. Rev. Phys. Chem. 43, 497–523 (1992).
Tully, J. C. Perspective: nonadiabatic dynamics theory. J. Chem. Phys. 137, 22A301 (2012).
Egorova, D. Self-analysis of coherent oscillations in time-resolved optical signals. J. Phys. Chem. A 118, 10259–10267 (2014).
Farfan, C. A. & Turner, D. B. Interference among multiple vibronic modes in two-dimensional electronic spectroscopy. Mathematics 8, 157 (2020).
Womick, J. M. & Moran, A. M. Vibronic enhancement of exciton sizes and energy transport in photosynthetic complexes. J. Phys. Chem. B 115, 1347–1356 (2011).
Tiwari, V., Peters, W. K. & Jonas, D. M. Electronic energy transfer through non-adiabatic vibrational-electronic resonance. I. Theory for a dimer. J. Chem. Phys. 147, 154308 (2017).
Tiwari, V. & Jonas, D. M. Electronic energy transfer through non-adiabatic vibrational-electronic resonance. II. 1D spectra for a dimer. J. Chem. Phys. 148, 084308 (2018).
Kim, P. W. et al. Femtosecond photodynamics of the red/green cyanobacteriochrome NpR6012g4 from Nostoc punctiforme. 1. Forward dynamics. Biochemistry 51, 608–618 (2012).
Sanchez-Galvez, A. et al. Ultrafast radiationless deactivation of organic dyes: evidence for a two-state two-mode pathway in polymethine cyanines. J. Am. Chem. Soc. 122, 2911–2924 (2000).
Bonačić-Koutecký, V., Koutecký, J. & Michl, J. Neutral and charged biradicals, zwitterions, funnels in S1, and proton translocation: their role in photochemistry, photophysics and vision. Angew. Chem. Int. Ed. 26, 170–189 (1987).
Michl, J. & Bonačić-Koutecký, V. Electronic Aspects of Organic Photochemistry (Wiley, 1990).
Klessinger, M. & Michl, J. Excited States and Photochemistry of Organic Molecules (VCH, 1995).
Levine, B. G. & Martínez, T. J. Isomerization through conical intersections. Annu. Rev. Phys. Chem. 58, 613–634 (2007).
Guo, H. & Yarkony, D. R. Accurate nonadiabatic dynamics. Phys. Chem. Chem. Phys. 18, 26335–26352 (2016).
Atchity, G. J., Xantheas, S. S. & Ruedenberg, K. Potential energy surfaces near intersections. J. Chem. Phys. 95, 1862–1876 (1991).
Jumper, C. C. et al. Intramolecular radiationless transitions dominate exciton relaxation dynamics. Chem. Phys. Lett. 599, 23–33 (2014).
Jumper, C. C., van Stokkum, I. H. M., Mirkovic, T. & Scholes, G. D. Vibronic wavepackets and energy transfer in cryptophyte light-harvesting complexes. J. Phys. Chem. B 122, 6328–6340 (2018).
Fleming, G. R. & Cho, M. Chromophore-solvent dynamics. Annu. Rev. Phys. Chem. 47, 109–134 (1996).
Ishizaki, A., Calhoun, T. R., Schlau-Cohen, G. S. & Fleming, G. R. Quantum coherence and its interplay with protein environments in photosynthetic electronic energy transfer. Phys. Chem. Chem. Phys. 12, 7319–7337 (2010).
Ghosh, S. et al. Excitation energy transfer by coherent and incoherent mechanisms in the peridinin-chlorophyll a protein. J. Phys. Chem. Lett. 8, 463–469 (2017).
Tilluck, R. W. et al. Interexciton nonradiative relaxation pathways in the peridinin-chlorophyll protein. Cell Rep. Phys. Sci. 2, 100380 (2021).
Brejc, K., Ficner, R., Huber, R. & Steinbacher, S. Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 Å resolution. J. Mol. Biol. 249, 424–440 (1995).
Kerfeld, C. A., Melnicki, M. R., Sutter, M. & Dominguez-Martin, M. A. Structure, function and evolution of the cyanobacterial orange carotenoid protein and its homologs. New Phytol. 215, 937–951 (2017).
Rosinski, J., Hainfeld, J. F., Rigbi, M. & Siegelman, H. W. Phycobilisome ultrastructure and chromatic adaptation in Fremyella diplosiphon. Ann. Bot. 47, 1–12 (1981).
Duerring, M., Schmidt, G. B. & Huber, R. Isolation, crystallization, crystal structure analysis and refinement of constitutive C-phycocyanin from the chromatically adapting cyanobacterium Fremyella diplosiphon at 1.66 Å resolution. J. Mol. Biol. 217, 577–592 (1991).
Gantt, E., Lipschultz, C. A., Grabowski, J. & Zimmerman, B. K. Phycobilisomes from blue-green and red algae: isolation criteria and dissociation characteristics. Plant Physiol. 63, 615–620 (1979).
Aráoz, R. & Häder, D.-P. Ultraviolet radiation induces both degradation and synthesis of phycobilisomes in Nostoc sp.: a spectroscopic and biochemical approach. FEMS Microbiol. Ecol. 23, 301–313 (1997).
Cobley, J. G. et al. Construction of shuttle plasmids which can be efficiently mobilized from Escherichia coli into the chromatically adapting cyanobacterium, Fremyella diplosiphon. Plasmid 30, 90–105 (1993).
Gurchiek, J. K. et al. Fluorescence anisotropy detection of barrier crossing and ultrafast conformational dynamics in the S2 state of β-carotene. J. Phys. Chem. B 124, 9029–9046 (2020).
Tilluck, R. W. et al. Interexciton nonradiative relaxation pathways in the peridinin-chlorophyll protein. Cell Rep. Phys. Sci. 2, 100380 (2021).
Shim, S. H. & Zanni, M. T. How to turn your pump-probe instrument into a multidimensional spectrometer: 2D IR and Vis spectroscopies via pulse shaping. Phys. Chem. Chem. Phys. 11, 748–761 (2009).
Lozovoy, V. V., Pastirk, I. & Dantus, M. Multiphoton intrapulse interference 4: characterization and compensation of the spectral phase of ultrashort laser pulses. Opt. Lett. 29, 775–777 (2004).
DeLong, K. W., Trebino, R., Hunter, J. & White, W. E. Frequency-resolved optical gating with the use of second-harmonic generation. J. Opt. Soc. Am. B 11, 2206–2215 (1994).
Augulis, R. & Zigmantas, D. Two-dimensional electronic spectroscopy with double modulation lock-in detection: enhancement of sensitivity and noise resistance. Opt. Express 19, 13126–13133 (2011).
Sil, S. et al. Excitation energy transfer and vibronic coherence in intact phycobilisomes—multidimensional electronic spectroscopy dataset and MATLAB and Julia analysis code. Zenodo https://doi.org/10.5281/zenodo.6743715 (2022).
Acknowledgements
Work in the laboratory of W.F.B. was principally supported by grant award no. DE-SC0010847 from the Photosynthetic Systems Program of the Office of Basic Energy Sciences, US Department of Energy. Work in the laboratory of C.A.K. was supported by grant award no. DE-SC0020606 from the Photosynthetic Systems Program of the Office of Basic Energy Sciences, US Department of Energy. We thank D. Sheppard, S. Lechno-Yossef and H. Bao in the Kerfeld laboratory for their assistance with the cyanobacterial cultures and with the isolation of the phycobilisome samples.
Author information
Authors and Affiliations
Contributions
S.S., R.W.T., C.A.K. and W.F.B. conceived the study and organized the project, with C.A.K. and W.F.B. providing overall supervision. S.S., M.A.D.-M. and W.L. set up the cyanobacterial cultures and the growth conditions and isolated the phycobilisome samples. S.S., R.W.T., N.M.T.M. and W.F.B. designed the multidimensional spectroscopy experiments, with S.S. and N.M.T.M. responsible for setting up the experiments and recording the datasets presented in the study. S.S., N.M.T.M. and C.H.L. analysed the datasets and produced the plotted results. S.S., R.W.T. and N.M.T.M. performed the global analysis. S.S. and J.B.R. performed the fluorescence experiments and analysed the results. S.S., N.M.T.M., C.H.L. and W.F.B. contributed the overall interpretation of the experimental results. The draft manuscript was written by S.S. and W.F.B., with contributions from N.M.T.M. and C.A.K. The revised manuscripts were written by S.S. and W.F.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Gregory Scholes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Continuous fluorescence excitation–emission spectra from phycobilisome preparations from Fremyella diplosiphon.
a, for broken phycobilisomes, which were produced by suspension of the isolated intact phycobilisomes in a 80 mM phosphate buffer solution at pH 7; b, for intact phycobilisomes, as suspended in a 0.8 M phosphate buffer solution at pH 7.
Extended Data Fig. 2 Global and target model for the integral of the 15150–15500 cm−1 (645–660-nm) region of the excitation axis of the 2DES spectrum.
a, Four-compartment kinetic scheme, with the initial fractional excitations (in each box) and time constants. b, Time evolution of the populations. c, Evolution-associated difference spectra (EADS). d, Amplitude transients at five detection wavelengths, with the fitted global model (black curve) superimposed; the confidence intervals for each data point are indicated by bars. The T axis used in b and d is semilogarithmic, with the linear–log split at 100 fs. In d, the instrument-response function is shown for the 640-nm transient as a 12-fs Gaussian (gray dotted curve) centered at T = 0 fs.
Extended Data Fig. 3 Decay of excited-state absorption (ESA) and diagonal ground-state bleaching (GSB) in the 2DES spectra.
a, 2DES spectrum at T = 10 ps. b, Amplitude transients sampled at the marked coordinates in the 2DES spectrum with a transient fitted over the T > 40 fs range (solid black curve) composed of two exponential components, A(T) = a0 + ∑i ai exp(−T/τi), convoluted with a 12-fs Gaussian instrument-response function. For the diagonal (16.5,16.5) transient: a0 = −0.24, a1 = 4.9, τ1 = 1.4 ps, a2 = 9.8, τ2 = 38 ps; for the ESA transient (16.5,14.9): a0 = 3.2, a1 = −6.6, τ1 = 180 fs, a2 = −16, τ2 = 23 ps. The bars indicate 95% confidence intervals for the amplitudes. The delay T axis is semilogarithmic, with the linear/log split at 100 fs.
Extended Data Fig. 4 Oscillation maps for the Hann-windowed T = 50–500 fs range for the principal modulation components at 520, 830, 1300, and 1670 cm−1.
The non-oscillatory part of the 2DES signal was removed by subtracting an overdetermined 2D global model. Dashed lines in the oscillation maps are drawn evenly spaced from the diagonal of the spectrum by the selected modulation frequency. The black horizontal line along the detection axis marks the peak of the fluorescence oscillator-strength spectrum (Fig. 1f).
Extended Data Fig. 5 Oscillation maps for the Hann-windowed T = 50–500 fs range at 110, 270, 650, and 1050 cm−1.
The non-oscillatory part of the 2DES signal was removed by subtracting an overdetermined 2D global model. Dashed lines in the oscillation maps are drawn evenly spaced from the diagonal of the spectrum by the selected modulation frequency. The black horizontal line along the detection axis marks the peak of the fluorescence oscillator-strength spectrum (Fig. 1f).
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Supplementary Video 1
Video of the 2DES dataset.
Source data
Source Data Fig. 1
Fig. 1f: Model for linear absorption spectrum of phycobilisomes from Fremyella diplosiphon using component phycobiliprotein absorption spectra.
Source Data Fig. 3
Fig. 3c: Evolution associated difference spectra (EADS) from the global model for the 550–580 nm excitation region of the 2DES spectra.
Source Data Fig. 4
Fig. 4c: Oscillatory transients above and below the diagonal of the 2DES spectrum.
Source Data Fig. 5
Fig. 5a: FT spectra for transients shown in Fig. 3d after subtraction of the global model.
Source Data Fig. 6
Fig. 6d: Model energy levels for the phycobilisome.
Source Data Extended Data Fig. 2
Extended Data Fig. 2c: Evolution associated difference spectra (EADS) from the global model for the 646–660 nm excitation region of the 2DES spectra.
Source Data Extended Data Fig. 3
Extended Data Fig. 3b: Amplitude transients for the diagonal and ESA coordinates.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sil, S., Tilluck, R.W., Mohan T. M., N. et al. Excitation energy transfer and vibronic coherence in intact phycobilisomes. Nat. Chem. 14, 1286–1294 (2022). https://doi.org/10.1038/s41557-022-01026-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01026-8