Abstract
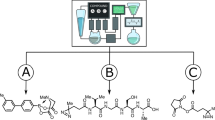
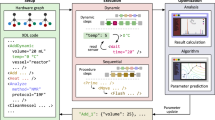
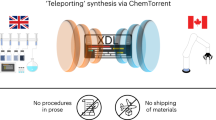
Robotic systems for synthetic chemistry are becoming more common, but they are expensive, fixed to a narrow set of reactions, and must be used within a complex laboratory environment. A portable system that could synthesize known molecules anywhere, on demand, and in a fully automated way, could revolutionize access to important molecules. Here we present a portable suitcase-sized chemical synthesis platform containing all the modules required for synthesis and purification. The system uses a chemical programming language coupled to a digital reactor generator to produce reactors and executable protocols based on text-based literature syntheses. Simultaneously, the platform generates a reaction pressure fingerprint, used to monitor processes within the reactors and remotely perform a protocol quality control. We demonstrate the system by synthesizing five small organic molecules, four oligopeptides and four oligonucleotides, in good yields and purities, with a total of 24,936 base steps executed over 329 h of platform runtime.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The Supplementary Information includes full details to reproduce this work. This consists of full details to reproduce the electronic and mechanical construction of the platform. The XDL files (.xdl), along with the respective graph (.json) and 3D reactor design (.stl), and full analytical data are provided, including our pneumatic fingerprint for the reactions, at https://doi.org/10.5281/zenodo.5248762.
Code availability
Code is available from https://doi.org/10.5281/zenodo.5248762.
References
Nicolaou, K. C. & Jamir, S. C. Classics in Total Synthesis III: Further Targets, Strategies, Methods (Wiley, 2011).
Nicolaou, K. C. & Sorensen, E. J. Classics in Total Synthesis: Targets, Strategies, Methods (Wiley, 1996).
Nicolaou, K. C. & Snyder, S. A. Classics in Total Synthesis II: More Targets, Strategies, Methods (Wiley, 2003).
Mehr, S. H. M., Craven, M., Leonov, A. I., Keenan, G. & Cronin, L. A universal system for digitization and automatic execution of the chemical synthesis literature. Science 370, 101–108 (2020).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Steiner, S. et al. Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363, eaav2211 (2019).
Angelone, D. et al. Convergence of multiple synthetic paradigms in a universally programmable chemical synthesis machine. Nat. Chem. 13, 63–69 (2021).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365, eaax1566 (2019).
Merrifield, R. B. Automated synthesis of peptides. Science 150, 178–185 (1965).
Alvarado-Urbina, G. et al. Automated synthesis of gene fragments. Science 214, 270–274 (1981).
Plante, O. J., Palmacci, E. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001).
Joseph, A. A., Pardo-Vargas, A. & Seeberger, P. H. Total synthesis of polysaccharides by automated glycan assembly. J. Am. Chem. Soc. 142, 8561–8564 (2020).
Jiang, T. et al. An integrated console for capsule-based, automated organic synthesis. Chem. Sci 12, 6977–6982 (2021).
Ley, S. V. & Baxendale, I. R. New tools and concepts for modern organic synthesis. Nat. Rev. Drug Discov. 1, 573–586 (2002).
Schotten, C., Leist, L. G. T., Semrau, A. L. & Browne, D. L. A machine-assisted approach for the preparation of follow-on pharmaceutical compound libraries. React. Chem. Eng. 3, 210–215 (2018).
Chatterjee, S., Guidi, M., Seeberger, P. H. & Gilmore, K. Automated radial synthesis of organic molecules. Nature 579, 379–384 (2020).
Britton, J. & Jamison, T. F. A unified continuous flow assembly-line synthesis of highly substituted pyrazoles and pyrazolines. Angew. Chem. Int. Ed. 56, 8823–8827 (2017).
Bédard, A.-C. et al. Reconfigurable system for automated optimization of diverse chemical reactions. Science 361, 1220–1225 (2018).
Ghislieri, D., Gilmore, K. & Seeberger, P. H. Chemical assembly systems: layered control for divergent, continuous, multistep syntheses of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 54, 678–682 (2015).
Zhang, P. et al. Advanced continuous flow platform for on-demand pharmaceutical manufacturing. Chem. Eur. J. 24, 2776–2784 (2018).
Hou, W. et al. Automatic generation of 3D-Printed reactionware for chemical synthesis digitization using ChemSCAD. ACS Cent. Sci. 7, 212–218 (2021).
Kitson, P. J. et al. Digitization of multistep organic synthesis in reactionware for on-demand pharmaceuticals. Science 359, 314–319 (2018).
Zalesskiy, S. S., Kitson, P. J., Frei, P., Bubliauskas, A. & Cronin, L. 3D designed and printed chemical generators for on demand reagent synthesis. Nat. Commun. 10, 5496 (2019).
Agnew, P. C. et al. A clinical evalutation of four antidepresant drugs. Am. J. Psychiatry 118, 160–162 (1961).
Youatt, J. A review of the action of isoniazid. Am. Rev. Respir. Dis. 99, 729–749 (1969).
Timmins, G. S. & Deretic, V. Mechanisms of action of isoniazid. Mol. Microbiol. 62, 1220–1227 (2006).
Zhang, Y., Dhandayuthapani, S. & Deretic, V. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc. Natl Acad. Sci. USA 93, 13212–13216 (1996).
Heilmann, L., Wacker, J. & Rath, W. Diagnosis and treatment of hypertensive disorders during pregnancy in Germany: results of a survey in German obstetric clinics. Geburtshilfe Frauenheilkd 64, 589–599 (2004).
Chakkath, T., Lavergne, S., Fan, T. M., Bunick, D. & Dirikolu, L. Alkylation and carbamylation effects of lomustine and its major metabolites and MGMT expression in canine cells. Vet. Sci. 2, 52–68 (2015).
Blaising, J., Polyak, S. J. & Pécheur, E.-I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 107, 84–94 (2014).
Acknowledgements
We thank D. Caramelli for producing the Supplementary Video and S. Rohrbach and M. Siauciulis for helpful comments on the manuscript. We gratefully acknowledge financial support from the EPSRC (grants nos. EP/L023652/1, EP/R020914/1, EP/S030603/1, EP/R01308X/1, EP/S017046/1 and EP/S019472/1), the ERC (project no. 670467 SMART-POM), the EC (project no. 766975 MADONNA) and DARPA (projects nos. W911NF-18-2-0036, W911NF-17-1-0316 and HR001119S0003). The views, opinions and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the US Government.
Author information
Authors and Affiliations
Contributions
L.C. invented the concept and devised the project and the digitization approach, with help from J.S.M., S.S.Z. and P.J.K. S.S.Z. developed the initial system design and built the first prototype together with J.S.M. W.H. carried out reactionware synthetic routes for the small organic molecules, and P.F. and H.W. helped with method development for the synthesis of oligopeptides and oligonucleotides. J.S.M. carried out all the automated synthesis and developed the necessary code for the platform. J.S.M. and P.J.K. wrote the paper, with help from L.C.
Corresponding author
Ethics declarations
Competing interests
The work described here has been filed as a patent GB 2213747.5 filed by the University of Glasgow.
Peer review
Peer review information
Nature Chemistry thanks Melodie Christensen, Kerry Gilmore and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Video 1
Animation of a typical literature to cartridge to synthesis process.
Supplementary Data 1
stl, .xdl, .json for all the syntheses executed in the portable platform.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manzano, J.S., Hou, W., Zalesskiy, S.S. et al. An autonomous portable platform for universal chemical synthesis. Nat. Chem. 14, 1311–1318 (2022). https://doi.org/10.1038/s41557-022-01016-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01016-w
This article is cited by
-
Reinvent 4: Modern AI–driven generative molecule design
Journal of Cheminformatics (2024)
-
An autonomous laboratory for the accelerated synthesis of novel materials
Nature (2023)