Abstract
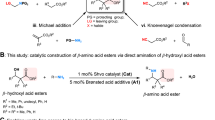
β-Amino acids are frequently found as important components in numerous biologically active molecules, drugs and natural products. In particular, they are broadly utilized in the construction of bioactive peptides and peptidomimetics, thanks to their increased metabolic stability. Despite the number of methodologies established for the preparation of β-amino acid derivatives, the majority of these methods require metal-mediated multistep manipulations of prefunctionalized substrates. Here we disclose a metal-free, energy-transfer enabled highly regioselective intermolecular aminocarboxylation reaction for the single-step installation of both amine and ester functionalities into alkenes or (hetero)arenes. A bifunctional oxime oxalate ester was developed to simultaneously generate C-centred ester and N-centred iminyl radicals. This mild method features a remarkably broad substrate scope (up to 140 examples) and excellent tolerance of sensitive functional groups, and substrates that range from the simplest ethylene to complex (hetero)arenes can participate in the reaction, thus offering a general and practical access to β-amino acid derivatives.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Materials and methods, experimental procedures, mechanistic studies, computational studies, sensitivity assessment and NMR spectra are available in the Supplementary Information. CIF crystallographic data files and xyz coordinates of the optimized structures are available as Supplementary Files. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2113934 (1), 2113935 (S1), 2115949 (S3) and 2114975 (138). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Amino Acid Market Research Report by Product Type, Source, Application, and Region-Global Forecast to 2026—Cumulative Impact of COVID-19 (Research And Markets, accessed 30 October 2021); https://www.researchandmarkets.com/reports/4905142/amino-acid-market-research-report-by-product#tag-pos-3
Cardillo, G. & Tomasini, C. Asymmetric synthesis of ß-amino acids and α-substituted β-amino acids. Chem. Soc. Rev. 25, 117–128 (1996).
Juaristi, E. & Soloshonok, V. A. Enantioselective Synthesis of β-Amino Acids (Wiley, 2005).
Kudo, F., Miyanaga, A. & Eguchi, T. Biosynthesis of natural products containing β-amino acids. Nat. Prod. Rep. 31, 1056–1073 (2014).
Spiteller, P. Amino Acids, Peptides and Proteins in Organic Chemistry (Wiley, 2009).
Cabrele, C., Martinek, T. A., Reiser, O. & Berlicki, L. Peptides containing β‑amino acid patterns: challenges and successes in medicinal chemistry. J. Med. Chem. 57, 9718–9739 (2014).
Seebach, D. & Gardiner, J. β-peptidic peptidomimetics. Acc. Chem. Res 41, 1366–1375 (2008).
Wang, L., Mao, Y., Wang, Z., Ma, H. & Chen, T. Advances in biotechnological production of β‑alanine. World J. Microbiol. Biotechnol. 37, 79 (2021).
Zrenner, R. H. et al. A functional analysis of the pyrimidine catabolic pathway in arabidopsis. New Phytol. 183, 117–132 (2009).
Miltenberger, K. Ullmann’s Encyclopedia of Industrial Chemistry—Hydroxycarboxylic Acids, Aliphatic (Wiley, 2000).
Weiner, B., Szymański, W., Janssen, D. B., Minnaard, A. J. & Feringa, B. L. Recent advances in the catalytic asymmetric synthesis of β-amino acids. Chem. Soc. Rev. 39, 1656–1691 (2010).
Rulev, R. Y. Aza-Michael reaction: achievements and prospects. Russ. Chem. Rev. 80, 197–218 (2011).
Qian, B., Chen, S., Wang, T., Zhang, X. & Bao, H. Iron-catalyzed carboamination of olefins: synthesis of amines and disubstituted β‑amino acids. J. Am. Chem. Soc. 139, 13076–13082 (2017).
Bruneau, C., Renaud, J.-L. & Jerphagnon, T. Synthesis of β-amino acid derivatives via enantioselective hydrogenation of β-substituted-β-(acylamino)acrylates. Coord. Chem. Rev. 252, 532–544 (2008).
Kobayashi, S., Mori, Y., Fossey, J. S. & Salter, M. M. Catalytic enantioselective formation of C–C bonds by addition to imines and hydrazones: a ten-year update. Chem. Rev. 111, 2626–2704 (2011).
Meyer, C. C., Ortiz, E. & Krische, M. J. Catalytic reductive aldol and Mannich reactions of enone, acrylate, and vinyl heteroaromatic pronucleophiles. Chem. Rev. 120, 3721–3748 (2020).
Podlech, J. & Seebach, D. The Arndt–Eistert reaction in peptide chemistry: a facile access to homopeptides. Angew. Chem. Int. Ed. 34, 471–472 (1995).
Cheng, J., Qi, X., Li, M., Chen, P. & Liu, G. Palladium-catalyzed intermolecular aminocarbonylation of alkenes: efficient access of β‑amino acid derivatives. J. Am. Chem. Soc. 137, 2480–2483 (2015).
Davies, J. et al. Ni-catalyzed carboxylation of aziridines en route to β‑amino acids. J. Am. Chem. Soc. 143, 4949–4954 (2021).
Xuan, J. & Xiao, W.-J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 51, 6828–6838 (2012).
Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Stephenson, C. R. J., Yoon, T. & MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry (Wiley, 2018).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Zhou, Q.-Q., Zou, Y.-Q., Lu, L.-Q. & Xiao, W.-J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew. Chem. Int. Ed. 58, 1586–1604 (2019).
Strieth-Kalthoff, F. & Glorius, F. Triplet energy transfer photocatalysis: unlocking the next level. Chem 6, 1888–1903 (2020).
Teders, M. et al. The energy-transfer-enabled biocompatible disulfide–ene reaction. Nat. Chem. 10, 981–988 (2018).
Ma, J. et al. Photochemical intermolecular dearomative cycloaddition of bicyclic azaarenes with alkenes. Science 371, 1338–1345 (2021).
Huang, H.-M., Bellotti, P., Ma, J., Dalton, T. & Glorius, F. Bifunctional reagents in organic synthesis. Nat. Rev. Chem. 5, 301–321 (2021).
Pitzer, L., Schäfers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed. 58, 8572–8576 (2019).
Silverman, R. B. From basic science to blockbuster drug: the discovery of lyrica. Angew. Chem. Int. Ed. 47, 3500–3504 (2008).
Purdy, R. H., Morrow, A. L., Moore, P. H. & Paul, S. M. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl Acad. Sci. USA 88, 4553–4557 (1991).
Wille, U. Radical cascades initiated by intermolecular radical addition to alkynes and related triple bond systems. Chem. Rev. 113, 813–853 (2013).
Xiong, T. & Zhang, Q. Recent advances in the direct construction of enantioenriched stereocenters through addition of radicals to internal alkenes. Chem. Soc. Rev. 50, 8857–8873 (2021).
Monos, T. M., McAtee, R. C. & Stephenson, C. R. J. Arylsulfonylacetamides as bifunctional reagents for alkene aminoarylation. Science 361, 1369–1373 (2018).
Constantin, T. et al. Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides. Science 367, 1021–1026 (2020).
Bunescu, A., Abdelhamid, Y. & Gaunt, M. J. Multicomponent alkene azido-arylation by anion-mediated dual catalysis. Nature 598, 597–603 (2021).
Fische, H. The persistent radical effect: a principle for selective radical reactions and living radical polymerizations. Chem. Rev. 101, 3581–3610 (2001).
Leifert, D. & Studer, A. The persistent radical effect in organic synthesis. Angew. Chem. Int. Ed. 59, 74–108 (2020).
Su, Y.-L. et al. Radical-mediated strategies for the functionalization of alkenes with diazo compounds. J. Am. Chem. Soc. 142, 13846–13855 (2020).
Patra, T., Bellotti, P. & Glorius, F. Photosensitized intermolecular carboimination of alkenes through persistent radical effect. Angew. Chem. Int. Ed. 59, 3172–3177 (2020).
Karageorgis, G., Warriner, S. & Nelson, A. Efficient discovery of bioactive scaffolds by activity-directed synthesis. Nat. Chem. 6, 872–876 (2014).
Zhang, X. et al. A general strategy for synthesis of cyclophane-braced peptide macrocycles via palladium-catalysed intramolecular sp3 C−H arylation. Nat. Chem. 10, 540–548 (2018).
Khan, M. U. et al. Synthesis and characterization of metabolites and potential impurities of balsalazide disodium, an anti-inflammatory drug. Synth. Commun. 40, 2241–2253 (2010).
Desai, A. A. Sitagliptin manufacture: a compelling tale of green chemistry, process intensification, and industrial asymmetric catalysis. Angew. Chem. Int. Ed. 50, 1974–1976 (2011).
Nikitas, N. F., Gkizis, P. L. & Kokotos, C. G. Thioxanthone: a powerful photocatalyst for organic reactions. Org. Biomol. Chem. 19, 5237–5253 (2021).
Kudisch, M., Lim, C.-H., Thordarson, P. & Miyake, G. M. Energy transfer to Ni–amine complexes in dual catalytic, light-driven C–N cross-coupling reactions. J. Am. Chem. Soc. 141, 19479–19486 (2019).
Patra, T., Mukherjee, S., Ma, J., Strieth-Kalthoff, F. & Glorius, F. Visible-light-photosensitized aryl and alkyl decarboxylative functionalization reactions. Angew. Chem. Int. Ed. 58, 10514–10520 (2019).
Patra, T., Das, M., Daniliuc, C. G. & Glorius, F. Metal-free, photosensitized oxyimination of unactivated alkenes with bifunctional oxime carbonates. Nat. Catal. 4, 54–61 (2021).
Acknowledgements
We thank J. Ma, X. Zhang, X. Yu, T. Hu (all WWU) and S. Chang (KAIST) for helpful assistance and discussion. Generous financial support from the Alexander von Humboldt Foundation and the Deutsche Forschungsgemeinschaft (SFB 858) is gratefully acknowledged. H.K. thanks the Institute for Basic Science (IBS-R010-D1) in the Republic of Korea for financial support.
Author information
Authors and Affiliations
Contributions
F.G. and G.T. conceived the project. G.T., M.D. and P.B. performed the synthetic experiments. H.K. performed the density functional theory calculations. C.D. analysed the X-ray structures. G.T., M.D., H.K. and F.G. supervised the research and wrote the manuscript with contributions from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Gabriela Oksdath-Mansilla and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–14 and Tables 1–4. Experimental data, synthesis and characterization data, supplementary discussion, computational and procedural details, x-ray crystallographic data, NMR spectra.
Supplementary Data 1
Crystallographic data for compound 1; CCDC reference 2113934.
Supplementary Data 2
Crystallographic data for compound 138; CCDC reference 2114975.
Supplementary Data 3
Crystallographic data for compound S1; CCDC reference 2113935.
Supplementary Data 4
Crystallographic data for compound S3; CCDC reference 2115949.
Supplementary Data 5
Cartesian coordinates for all calculated structures.
Rights and permissions
About this article
Cite this article
Tan, G., Das, M., Keum, H. et al. Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes. Nat. Chem. 14, 1174–1184 (2022). https://doi.org/10.1038/s41557-022-01008-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01008-w
This article is cited by
-
The impact of UV light on synthetic photochemistry and photocatalysis
Nature Chemistry (2024)
-
Modular access to alkylgermanes via reductive germylative alkylation of activated olefins under nickel catalysis
Nature Communications (2023)
-
Cobalt-catalyzed aminoalkylative carbonylation of alkenes toward direct synthesis of γ-amino acid derivatives and peptides
Nature Communications (2023)
-
Dearomative triple elementalization of quinolines driven by visible light
Nature Communications (2023)
-
Photoinduced radical–ionic dihalogen transfer to carbon–carbon multiple bonds using oxime-based surrogates
Nature Synthesis (2023)