Abstract
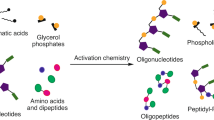
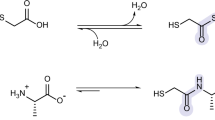
Nucleosides are essential to the emergence of life, and so their synthesis is a key challenge for prebiotic chemistry. Although amino-nucleosides have enhanced reactivity in water compared with ribonucleosides, they are assumed to be prebiotically irrelevant due to perceived difficulties with their selective formation. Here we demonstrate that 3′-amino-TNA nucleosides (TNA, threose nucleic acid) are formed diastereoselectively and regiospecifically from prebiotic feedstocks in four high-yielding steps. Phosphate provides an unexpected resolution, leading to spontaneous purification of the genetically relevant threo-isomer. Furthermore, 3′-amino-TNA nucleosides are shown to be phosphorylated directly in water, under mild conditions with cyclic trimetaphosphate, forming a nucleoside triphosphate (NTP) in a manner not feasible for canonical nucleosides. Our results suggest 3′-amino-TNA nucleosides may have been present on the early Earth, and the ease with which these NTPs form, alongside the inherent selectivity for the Watson–Crick base-pairing threo-monomer, warrants further study of the role they could play during the emergence of life.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data (experimental procedures and characterization data) supporting the findings of this study are available within the article and its Supplementary Information. Crystallographic data for the threo-7·H3PO4 reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2087673. Copies of the data can be obtained free of charge from CCDC via https://www.ccdc.cam.ac.uk/structures/.
References
Szostak, J. W. The narrow road to the deep past: in search of the chemistry of the origin of life. Angew. Chem. Int. Ed. 56, 11037–11043 (2017).
Robertson, M. P. & Joyce, G. F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 4, a003608 (2012).
Schöning, K.-U. et al. The -L-threofuranosyl-(3′→2′)-oligonucleotide system (‘TNA’): synthesis and pairing properties. Helv. Chim. Acta 85, 4111–4153 (2002).
Bhowmik, S. & Krishnamurthy, R. The role of sugar-backbone heterogeneity and chimeras in the simultaneous emergence of RNA and DNA. Nat. Chem. 11, 1009–1018 (2019).
Joyce, G. F., Schwartz, A. W., Miller, S. L. & Orgel, L. E. The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc. Natl Acad. Sci. USA 84, 4398–4402 (1987).
Fialho, D. M., Roche, T. P. & Hud, N. V. Prebiotic syntheses of noncanonical nucleosides and nucleotides. Chem. Rev. 120, 4806–4830 (2020).
Becker, S., Schneider, C., Crisp, A. & Carell, T. Non-canonical nucleosides and chemistry of the emergence of life. Nat. Commun. 9, 5174 (2018).
Hud, N. V. Searching for lost nucleotides of the pre-RNA World with a self-refining model of early Earth. Nat. Commun. 9, 5171 (2018).
Probst, A. V., Dunleavy, E. & Almouzni, G. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 10, 192–206 (2009).
Beier, M., Reck, F., Wagner, T., Krishnamurthy, R. & Eschenmoser, A. Chemical etiology of nucleic acid structure: comparing pentopyranosyl-(2′→4′) oligonucleotides with RNA. Science 283, 699–703 (1999).
Colville, B. W. F. & Powner, M. W. Selective prebiotic synthesis of α‐threofuranosyl cytidine by photochemical anomerization. Angew. Chem. Int. Ed. 60, 10526–10530 (2021).
Wu, X., Guntha, S., Ferencic, M., Krishnamurthy, R. & Eschenmoser, A. Base-pairing systems related to TNA: α-threofuranosyl oligonucleotides containing phosphoramidate linkages. Org. Lett. 4, 1279–1282 (2002).
Wang, Y. et al. A threose nucleic acid enzyme with RNA ligase activity. J. Am. Chem. Soc. 143, 8154–8163 (2021).
Yu, H., Zhang, S. & Chaput, J. C. Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor. Nat. Chem. 4, 183–187 (2012).
Eschenmoser, A. The TNA-family of nucleic acid systems: properties and prospects. Orig. Life Evol. Biosph. 34, 277–306 (2004).
Whitaker, D. & Powner, M. W. Prebiotic nucleic acids need space to grow. Nat. Commun. 9, 5172 (2018).
Islam, S. & Powner, M. W. Prebiotic systems chemistry: complexity overcoming clutter. Chem 2, 470–501 (2017).
Yadav, M., Kumar, R. & Krishnamurthy, R. Chemistry of abiotic nucleotide synthesis. Chem. Rev. 120, 4766–4805 (2020).
Becker, S. et al. Unified prebiotically plausible synthesis of pyrimidine and purine RNA ribonucleotides. Science 366, 76–82 (2019).
Powner, M. W., Gerland, B. & Sutherland, J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009).
Stairs, S. et al. Divergent prebiotic synthesis of pyrimidine and 8-oxo-purine ribonucleotides. Nat. Commun. 8, 15270 (2017).
Islam, S., Bučar, D.-K. & Powner, M. W. Prebiotic selection and assembly of proteinogenic amino acids and natural nucleotides from complex mixtures. Nat. Chem. 9, 584–589 (2017).
Ponnamperuma, C. & Woeller, F. α-Aminonitriles formed by an electric discharge through a mixture of anhydrous methane and ammonia. Curr. Mod. Biol. 1, 156–158 (1967).
Patel, B. H., Percivalle, C., Ritson, D. J., Duffy, C. D. & Sutherland, J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015).
Canavelli, P., Islam, S. & Powner, M. W. Peptide ligation by chemoselective aminonitrile coupling in water. Nature 571, 546–549 (2019).
Foden, C. S. et al. Prebiotic synthesis of cysteine peptides that catalyze peptide ligation in neutral water. Science 370, 865–869 (2020).
Powner, M. W., Sutherland, J. D. & Szostak, J. W. Chemoselective multicomponent one-pot assembly of purine precursors in water. J. Am. Chem. Soc. 132, 16677–16688 (2010).
Hein, J. E., Tse, E. & Blackmond, D. G. A route to enantiopure RNA precursors from nearly racemic starting materials. Nat. Chem. 3, 704–706 (2011).
Powner, M. W. & Sutherland, J. D. Phosphate-mediated interconversion of ribo- and arabino-configured prebiotic nucleotide intermediates. Angew. Chem. Int. Ed. 49, 4641–4643 (2010).
Ashe, K. et al. Selective prebiotic synthesis of phosphoroaminonitriles and aminothioamides in neutral water. Commun. Chem. 2, 23 (2019).
Moutou, G. et al. Equilibrium of α-aminoacetonitrile formation from formaldehyde, hydrogen cyanide and ammonia in aqueous solution: industrial and prebiotic significance. J. Phys. Org. Chem. 8, 721–730 (1995).
Taillades, J. & Commeyras, A. Systemes de Strecker et apparentes—I Etude de la decomposition en solution aqueuse des α-alcoyl-aminonitriles tertiaires. Mécanisme d’élimination du groupement nitrile. Tetrahedron 30, 127–132 (1974).
Taillades, J. et al. N-carbamoyl-α-amino acids rather than free α-amino acids formation in the primitive hydrosphere: a novel proposal for the emergence of prebiotic peptides. Orig. Life Evol. Biosph. 28, 61–77 (1998).
Springsteen, G. & Joyce, G. F. Selective derivatization and sequestration of ribose from a prebiotic mix. J. Am. Chem. Soc. 126, 9578–9583 (2004).
Ferris, J. P., Sanchez, R. A. & Orgel, L. E. Studies in prebiotic synthesis: III. Synthesis of pyrimidines from cyanoacetylene and cyanate. J. Mol. Biol. 33, 693–704 (1968).
Ni, G. et al. Review of α-nucleosides: from discovery, synthesis to properties and potential applications. RSC Adv. 9, 14302–14320 (2019).
Sanchez, R. A. & Orgel, L. E. Studies in prebiotic synthesis. V. Synthesis and photoanomerization of pyrimidine nucleosides. J. Mol. Biol. 47, 531–543 (1970).
Xu, J. et al. A prebiotically plausible synthesis of pyrimidine β-ribonucleosides and their phosphate derivatives involving photoanomerization. Nat. Chem. 9, 303–309 (2017).
Grosjean, H., de Crécy-Lagard, V. & Marck, C. Deciphering synonymous codons in the three domains of life: co-evolution with specific tRNA modification enzymes. FEBS Lett. 584, 252–264 (2010).
Ajitkumar, P. & Cherayil, J. D. Thionucleosides in transfer ribonucleic acid: diversity, structure, biosynthesis and function. Microbiol. Rev. 52, 103–113 (1988).
Testa, S. M., Disney, M. D., Turner, D. H. & Kierzek, R. Thermodynamics of RNA-RNA duplexes with 2- or 4-thiouridines: implications for antisense design and targeting a group I intron. Biochemistry 38, 16655–16662 (1999).
Heuberger, B. D., Pal, A., Del Frate, F., Topkar, V. V. & Szostak, J. W. Replacing uridine with 2-thiouridine enhances the rate and fidelity of nonenzymatic RNA primer extension. J. Am. Chem. Soc. 137, 2769–2775 (2015).
Prywes, N., Michaels, Y. S., Pal, A., Oh, S. S. & Szostak, J. W. Thiolated uridine substrates and templates improve the rate and fidelity of ribozyme-catalyzed RNA copying. Chem. Commun. 52, 6529–6532 (2016).
Ohkubo, A. et al. Formation of new base pairs between inosine and 5-methyl-2-thiocytidine derivatives. Org. Biomol. Chem. 10, 2008–2010 (2012).
Kim, S. C., O’Flaherty, D. K., Zhou, L., Lelyveld, V. S. & Szostak, J. W. Inosine, but none of the 8-oxo-purines, is a plausible component of a primordial version of RNA. Proc. Natl Acad. Sci. USA 115, 13318–13323 (2018).
Gibard, C., Bhowmik, S., Karki, M., Kim, E. K. & Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 10, 212–217 (2018).
Lohrmann, R. Formation of nucleoside 5′-polyphosphates from nucleotides and trimetaphosphate. J. Mol. Evol. 6, 237–252 (1975).
Yamagata, Y. Prebiotic formation of ADP and ATP from AMP, calcium phosphates and cyanate in aqueous solution. Orig. Life Evol. Biosph. 29, 511–520 (1999).
Feldmann, W. & Thilo, E. Zur Chemie der Kondensierten Phosphate und Arsenate. XXXVIII. Amidotriphosphat. Zeitschrift Anorg. Allg. Chem. 328, 113–126 (1964).
Yamagata, Y., Watanabe, H., Saitoh, M. & Namba, T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 352, 516–519 (1991).
Weimann, B. J., Lohrmann, R., Orgel, L. E., Schneider-Bernloehr, H. & Sulston, J. E. Template-directed synthesis with adenosine-5′-phosphorimidazolide. Science 161, 387–387 (1968).
Li, L. et al. Enhanced nonenzymatic RNA copying with 2-aminoimidazole activated nucleotides. J. Am. Chem. Soc. 139, 1810–1813 (2017).
Zielinski, W. S. & Orgel, L. E. Oligomerization of activated derivatives of 3′-amino-3′-deoxyguanosine on poly(C) and poly(dC) templates. Nucleic Acids Res. 13, 2469–2484 (1985).
Röthlingshöfer, M. et al. Chemical primer extension in seconds. Angew. Chem. Int. Ed. 47, 6065–6068 (2008).
Chen, J. J., Cai, X. & Szostak, J. W. N2′→P3′ phosphoramidate glycerol nucleic acid as a potential alternative genetic system. J. Am. Chem. Soc. 131, 2119–2121 (2009).
Blain, J. C., Ricardo, A. & Szostak, J. W. Synthesis and nonenzymatic template-directed polymerization of 2′-amino-2′-deoxythreose nucleotides. J. Am. Chem. Soc. 136, 2033–2039 (2014).
Zhou, L., O’Flaherty, D. K. & Szostak, J. W. Template-directed copying of RNA by non-enzymatic ligation. Angew. Chem. Int. Ed. 59, 15682–15687 (2020).
Hänle, E. & Richert, C. Enzyme-free replication with two or four bases. Angew. Chem. Int. Ed. 57, 8911–8915 (2018).
O’Flaherty, D. K., Zhou, L. & Szostak, J. W. Nonenzymatic template-directed synthesis of mixed-sequence 3′-NP-DNA up to 25 nucleotides long inside model protocells. J. Am. Chem. Soc. 141, 10481–10488 (2019).
Saffhill, R. Selective phosphorylation of the cis-2′,3′-diol of unprotected ribonucleosides with trimetaphosphate in aqueous solution. J. Org. Chem. 35, 2881–2883 (1970).
Mullen, L. B. & Sutherland, J. D. Formation of potentially prebiotic amphiphiles by reaction of β-hydroxy-n-alkylamines with cyclotriphosphate. Angew. Chem. Int. Ed. 46, 4166–4168 (2007).
Krishnamurthy, R., Guntha, S. & Eschenmoser, A. Regioselective α-phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed. 39, 2281–2285 (2000).
Rohatgi, R., Bartel, D. P. & Szostak, J. W. Nonenzymatic, template-directed ligation of oligoribonucleotides is highly regioselective for the formation of 3′−5′ phosphodiester bonds. J. Am. Chem. Soc. 118, 3340–3344 (1996).
Acknowledgements
The Leverhulme Trust (RPG-2019-214 MWP), the Simons Foundation (318881FY19 MWP) and the Engineering and Physical Sciences Research Council (EP/P020410/1 MWP) provided financial support. We thank K. Karu and M. Puchnarewicz (mass spectrometry), M. Corpinot and K. Bucar (crystallography) and A. E. Aliev (NMR spectroscopy) for support.
Author information
Authors and Affiliations
Contributions
M.W.P. conceived the research. M.W.P. and D.W. designed and analysed the experiments and wrote the manuscript. D.W. conducted the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Three-component coupling of aldehyde 1, oxazole 3 and aminonitrile 6e to yield amino-nucleotide precursor 7 in water.
1H NMR spectra [400 MHz, H2O/D2O (9:1), 7.6–5.2 ppm] to show the: a, formation of oxazoline 12e (R = sBu) after 2 h at room temperature and pH 4.5 and b, retro-Strecker of 12e at room temperature and pH 4.5 to form 7 after 5 days.
Supplementary information
Supplementary Information
Experimental detail, expanded reactions conditions and reagent compatibility studies, experimental data and NMR spectra, Supplementary figures and tables, X-ray crystallographic data, synthesis of chemical standards and characterization data.
Supplementary Data 1
Crystal structure of threo-7 H3PO4; CCDC reference 2087673.
Rights and permissions
About this article
Cite this article
Whitaker, D., Powner, M.W. Prebiotic synthesis and triphosphorylation of 3′-amino-TNA nucleosides. Nat. Chem. 14, 766–774 (2022). https://doi.org/10.1038/s41557-022-00982-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00982-5
This article is cited by
-
Making nucleic acid monomers
Nature Chemistry (2022)