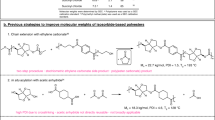
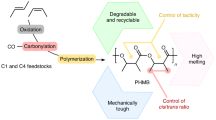
Abstract
Carbon dioxide is inexpensive and abundant, and its prevalence as waste makes it attractive as a sustainable chemical feedstock. Although there are examples of copolymerizations of CO2 with high-energy monomers, the direct copolymerization of CO2 with olefins has not been reported. Here an alternative route to functionalizable, recyclable polyesters derived from CO2, butadiene and hydrogen via an intermediary lactone, 3-ethyl-6-vinyltetrahydro-2H-pyran-2-one, is described. Catalytic ring-opening polymerization of the lactone by 1,5,7-triazabicyclo[4.4.0]dec-5-ene yields polyesters with molar masses up to 13.6 kg mol−1 and pendent vinyl side chains that can undergo post-polymerization functionalization. The polymer has a low ceiling temperature of 138 °C, allowing for facile chemical recycling, and is inherently biodegradable under aerobic aqueous conditions (OECD-301B protocol). These results show that a well-defined polyester can be derived from CO2, olefins and hydrogen, expanding access to new polymer feedstocks that were once considered unfeasible.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All primary data files63 are available free of charge from the Data Repository for the University of Minnesota at https://doi.org/10.13020/sy3d-cf59.
References
Omae, I. Recent developments in carbon dioxide utilization for the production of organic chemicals. Coord. Chem. Rev. 256, 1384–1405 (2012).
Sakakura, T., Choi, J. C. & Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 107, 2365–2387 (2007).
Grignard, B., Gennen, S., Jérôme, C., Kleij, A. W. & Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 48, 4466–4514 (2019).
Artz, J. et al. Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem. Rev. 118, 434–504 (2018).
Dabral, S. & Schaub, T. The use of carbon dioxide (CO2) as a building block in organic synthesis from an industrial perspective. Adv. Synth. Catal. 361, 223–246 (2019).
Khoo, R. S. H., Luo, H.-K., Braunstein, P. & Hor, T. S. A. Transformation of CO2 to value-added materials. J. Mol. Eng. Mater. 3, 1540007 (2015).
Zhu, Y., Romain, C. & Williams, C. K. Sustainable polymers from renewable resources. Nature 540, 354–362 (2016).
Price, C. J., Jesse, B., Reich, E. & Miller, S. A. Thermodynamic and kinetic considerations in the copolymerization of ethylene and carbon dioxide. Macromolecules 39, 2751–2756 (2006).
Musco, A., Perego, C. & Tartiari, V. Telomerization reactions of butadiene and CO2 catalyzed by phosphine Pd(0) complexes: (E)−2-ethylideneheptden-5-olide and octadienyl esters of 2-ethylidenehepta-4,6-dienoic acid. Inorg. Chim. Acta 28, L147–L148 (1978).
Inoue, Y., Sasaki, Y. & Hashimoto, H. Incorporation of CO2 in butadiene dimerization catalyzed by palladium complexes. Formation of 2-ethylidene-5-hepten-4-olide. Bull. Chem. Soc. Jpn 51, 2375–2378 (1978).
Braunstein, P., Matt, D. & Nobel, D. Carbon dioxide activation and catalytic lactone synthesis by telomerization of butadiene and CO2. J. Am. Chem. Soc. 110, 3207–3212 (1988).
Behr, A. & Juszak, K. D. Palladium-catalyzed reaction of butadiene and carbon dioxide. J. Organomet. Chem. 255, 263–268 (1983).
Sharif, M., Jackstell, R., Dastgir, S., Al-Shihi, B. & Beller, M. Efficient and selective palladium-catalyzed telomerization of 1,3-butadiene with carbon dioxide. ChemCatChem 9, 542–546 (2017).
Balbino, J. M., Dupont, J. & Bayón, J. C. Telomerization of 1,3-butadiene with carbon dioxide: a highly efficient process for δ-lactone generation. ChemCatChem 10, 206–210 (2018).
Song, J. et al. Selective synthesis of δ-lactone via palladium nanoparticles-catalyzed telomerization of CO2 with 1,3-butadiene. Tetrahedron Lett. 57, 3163–3166 (2016).
Behr, A. & Henze, G. Use of carbon dioxide in chemical syntheses via a lactone intermediate. Green Chem. 13, 25–39 (2011).
Haack, V., Dinjus, E. & Pitter, S. Synthesis of polymers with an intact lactone ring structure in the main chain. Angew. Makromol. Chem. 257, 19–22 (1998).
Hardouin Duparc, V., Shakaroun, R. M., Slawinski, M., Carpentier, J. F. & Guillaume, S. M. Ring-opening (co)polymerization of six-membered substituted ë-valerolactones with alkali metal alkoxides. Eur. Polym. J. 134, 109858 (2020).
Sajjad, H., Prebihalo, E. A., Tolman, W. B. & Reineke, T. M. Ring opening polymerization of β-acetoxy-δ-methylvalerolactone, a triacetic acid lactone derivative. Polym. Chem. 12, 6724–6730 (2021).
Schneiderman, D. K. & Hillmyer, M. A. Aliphatic polyester block polymer design. Macromolecules 49, 2419–2428 (2016).
Olsén, P., Odelius, K. & Albertsson, A. C. Thermodynamic presynthetic considerations for ring-opening polymerization. Biomacromolecules 17, 699–709 (2016).
Wheeler, O. H. & Granell, E. E. Solvolysis of substituted γ-butrolactones and δ-valerolactones. J. Org. Chem. 701, 1959–1961 (1964).
Nakano, R., Ito, S. & Nozaki, K. Copolymerization of carbon dioxide and butadiene via a lactone intermediate. Nat. Chem. 6, 325–331 (2014).
Tang, S., Zhao, Y. & Nozaki, K. Accessing divergent main-chain-functionalized polyethylenes via copolymerization of ethylene with a CO2/butadiene-derived lactone. J. Am. Chem. Soc. 143, 17953–17957 (2021).
Liu, M., Sun, Y., Liang, Y. & Lin, B. L. Highly efficient synthesis of functionalizable polymers from a CO2/1,3-butadiene-derived lactone. ACS Macro Lett. 6, 1373–1378 (2017).
Yue, S. et al. Ring-opening polymerization of CO2-based disubstituted δ-valerolactone toward sustainable functional polyesters. ACS Macro Lett. 10, 1055–1060 (2021).
Espinosa, L. D. G., Williams-Pavlantos, K., Turney, K. M., Wesdemiotis, C. & Eagan, J. M. Degradable polymer structures from carbon dioxide and butadiene. ACS Macro Lett. 10, 1254–1259 (2021).
Sugiura, M., Sato, N., Kotani, S. & Nakajima, M. Lewis base-catalyzed conjugate reduction and reductive aldol reaction of α,β-unsaturated ketones using trichlorosilane. Chem. Commun. 2, 4309–4311 (2008).
Behr, A. & Brehme, V. A. Bimetallic-catalyzed reduction of carboxylic acids and lactones to alcohols and diols. Adv. Synth. Catal. 344, 525–532 (2002).
Hudlicky, T., Sinai-Zingde, G. & Natchus, M. G. Selective reduction of α,β-unsaturated esters in the presence of olefins. Tetrahedron Lett. 28, 5287–5290 (1987).
Makiguchi, K., Satoh, T. & Kakuchi, T. Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone. Macromolecules 44, 1999–2005 (2011).
Delcroix, D. et al. Phosphoric and phosphoramidic acids as bifunctional catalysts for the ring-opening polymerization of ε-caprolactone: a combined experimental and theoretical study. Polym. Chem. 2, 2249–2256 (2011).
Dove, A. P. Organic catalysis for ring-opening polymerization. ACS Macro Lett. 1, 1409–1412 (2012).
Thomas, C. & Bibal, B. Hydrogen-bonding organocatalysts for ring-opening polymerization. Green Chem. 16, 1687–1699 (2014).
Chuma, A. et al. The reaction mechanism for the organocatalytic ring-opening polymerization of l-lactide using a guanidine-based catalyst: hydrogen-bonded or covalently bound? J. Am. Chem. Soc. 130, 6749–6754 (2008).
Pratt, R. C., Lohmeijer, B. G. G., Long, D. A., Waymouth, R. M. & Hedrick, J. L. Triazabicyclodecene: a simple bifunctional organocatalyst for acyl transfer and ring-opening polymerization of cyclic esters. J. Am. Chem. Soc. 128, 4556–4557 (2006).
Simón, L. & Goodman, J. M. The mechanism of TBD-catalyzed ring-opening polymerization of cyclic esters. J. Org. Chem. 72, 9656–9662 (2007).
Whelan, D. in Brydson’s Plastic Materials 8th edn (ed. Gilbert, M.) Ch. 24 (Butterworth-Heinemann, 2017).
Wanamaker, C. L., O’Leary, L. E., Lynd, N. A., Hillmyer, M. A. & Tolman, W. B. Renewable-resource thermoplastic elastomers based on polylactide and polymenthide. Biomacromolecules 8, 3634–3640 (2007).
Lin, B. & Waymouth, R. M. Urea anions: simple, fast and selective catalysts for ring-opening polymerizations. J. Am. Chem. Soc. 139, 1645–1652 (2017).
Anslyn, E. V. & Dougherty, D. A. in Modern Physical Organic Chemistry Ch. 2 (University Science Books, 2006).
Hong, M. & Chen, E. Y. X. Future directions for sustainable polymers. Trends Chem. 1, 148–151 (2019).
Tang, X. & Chen, E. Y. X. Toward infinitely recyclable plastics derived from renewable cyclic esters. Chem 5, 284–312 (2019).
Fagnani, D. E. et al. 100th anniversary of macromolecular science viewpoint: redefining sustainable polymers. ACS Macro Lett. 10, 41–53 (2021).
Darensbourg, D. J., Wei, S.-H., Yeung, A. D. & Chadwick Ellis, W. An efficient method of depolymerization of poly(cyclopentene carbonate) to its comonomers: cyclopentene oxide and carbon dioxide. Macromolecules 46, 5850–5855 (2013).
Zhu, J. B., Watson, E. M., Tang, J. & Chen, E. Y. X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Abel, B. A., Snyder, R. L. & Coates, G. W. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science 789, 783–789 (2021).
OECD. Test No. 301: Ready Biodegradability, OECD Guidelines for the Testing of Chemicals Section 3 (OECD, 1992).
Ditzler, R. A. J. & Zhukhovitskiy, A. V. Sigmatropic rearrangements of polymer backbones: vinyl polymers from polyesters in one step. J. Am. Chem. Soc. 143, 20326–20331 (2021).
Rieger, J. et al. Versatile functionalization and grafting of poly(ε-caprolactone) by Michael-type addition. Chem. Commun. 2005, 274–276 (2005).
Tang, X. et al. The quest for converting biorenewable bifunctional α-methylene-γ-butyrolactone into degradable and recyclable polyester: controlling vinyl-addition/ring-opening/cross-linking pathways. J. Am. Chem. Soc. 138, 14326–14337 (2016).
Campos, L. M. et al. Development of thermal and photochemical strategies for thiol-ene click polymer functionalization. Macromolecules 41, 7063–7070 (2008).
Hauenstein, O., Agarwal, S. & Greiner, A. Bio-based polycarbonate as synthetic toolbox. Nat. Commun. 7, 11862 (2016).
Chanda, S. & Ramakrishnan, S. Poly(alkylene itaconate)s – an interesting class of polyesters with periodically located exo-chain double bonds susceptible to Michael addition. Polym. Chem. 6, 2108–2114 (2015).
Ohsawa, S., Morino, K., Sudo, A. & Endo, T. Synthesis of a reactive polyester bearing α,β-unsaturated ketone groups by anionic alternating copolymerization of epoxide and bicyclic bis(γ-butyrolactone) bearing isopropenyl group. Macromolecules 44, 1814–1820 (2011).
Huang, K. S. et al. Recent advances in antimicrobial polymers: a mini-review. Int. J. Mol. Sci. 17, 1578–1592 (2016).
Șucu, T. & Shaver, M. P. Inherently degradable cross-linked polyesters and polycarbonates: resins to be cheerful. Polym. Chem. 11, 6397–6421 (2020).
Brutman, J. P., De Hoe, G. X., Schneiderman, D. K., Le, T. N. & Hillmyer, M. A. Renewable, degradable and chemically recyclable cross-linked elastomers. Ind. Eng. Chem. Res. 55, 11097–11106 (2016).
Robert, T. & Friebel, S. Itaconic acid—a versatile building block for renewable polyesters with enhanced functionality. Green Chem. 18, 2922–2934 (2016).
Fournier, L., Rivera Mirabal, D. M. & Hillmyer, M. A. Toward sustainable elastomers from the grafting-through polymerization of lactone-containing polyester macromonomers. Macromolecules 55, 1003–1014 (2022).
Huang, J. et al. DAB-Pd-MAH: a versatile Pd(0) source for precatalyst formation, reaction screening and preparative-scale synthesis. ACS Catal. 11, 5636–5646 (2021).
Mango, L. A. & Lenz, R. W. Hydrogenation of unsaturated polymers with diimide. Die Makromol. Chem. 163, 13–36 (1973).
Rapagnani, R. M., Dunscomb, R. J., Fresh, A. A. & Tonks, I. A. Supporting data for tunable and recyclable polyesters from CO2 and butadiene (Data Repository for the University of Minnesota, 2021); https://doi.org/10.13020/sy3d-cf59
Acknowledgements
The funding for this work was provided by the NSF Center for Sustainable Polymers (no. CHE-1901635 to I.A.T.) at the University of Minnesota. Instrumentation for the University of Minnesota Chemistry NMR facility was supported by a grant through the National Institutes of Health (no. S10OD011952).
Author information
Authors and Affiliations
Contributions
R.M.R., R.J.D. and A.A.F. designed and performed all experiments and carried out data analysis. I.A.T. directed the research. R.M.R. and I.A.T. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
I.A.T. and R.M.R. are co-inventors on a provisional US patent covering the methods of polymerization and composition of matter presented in this work, filed through the University of Minnesota (application no. 63/156,135). R.J.D. and A.A.F. declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks James Eagan, Xufeng Ni and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Materials and Methods, Supplementary text, Figs. 1 to 35, Tables 1 to 5, references, OECD-301B report from Situ Biosciences
Rights and permissions
About this article
Cite this article
Rapagnani, R.M., Dunscomb, R.J., Fresh, A.A. et al. Tunable and recyclable polyesters from CO2 and butadiene. Nat. Chem. 14, 877–883 (2022). https://doi.org/10.1038/s41557-022-00969-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00969-2
This article is cited by
-
Biorenewable and circular polydiketoenamine plastics
Nature Sustainability (2023)
-
A recyclable polyester library from reversible alternating copolymerization of aldehyde and cyclic anhydride
Nature Communications (2023)
-
A facile approach towards high-performance poly(thioether-thioester)s with full recyclability
Science China Chemistry (2023)