Abstract
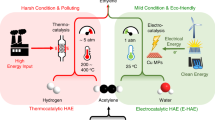
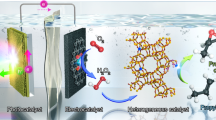
The production of polymers from ethylene requires the ethylene feed to be sufficiently purified of acetylene contaminant. Accomplishing this task by thermally hydrogenating acetylene requires a high temperature, an external feed of H2 gas and noble-metal catalysts. It is not only expensive and energy-intensive, but also prone to overhydrogenating to ethane. Here we report a photocatalytic system that reduces acetylene to ethylene with ≥99% selectivity under both non-competitive (no ethylene co-feed) and competitive (ethylene co-feed) conditions, and near 100% conversion under the latter industrially relevant conditions. Our system uses a molecular catalyst based on earth-abundant cobalt operating under ambient conditions and sensitized by either [Ru(bpy)3]2+ or an inexpensive organic semiconductor (metal-free mesoporous graphitic carbon nitride) under visible light. These features and the use of water as a proton source offer advantages over current hydrogenation technologies with respect to selectivity and sustainability.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data supporting the findings of this study are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use and fate of all plastics ever made. Sci. Adv. 3, 25–29 (2017).
Borodziński, A. & Bond, G. C. Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts. Part 1. Effect of changes to the catalyst during reaction. Catal. Rev. Sci. Eng. 48, 91–144 (2006).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320, 1320–1322 (2008).
Armbrüster, M. et al. Al13Fe4 as a low-cost alternative for palladium in heterogeneous hydrogenation. Nat. Mater. 11, 690–693 (2012).
Wang, Z. et al. Enhancement of alkyne semi-hydrogenation selectivity by electronic modification of platinum. ACS Catal. 10, 6763–6770 (2020).
Chen, K. J. et al. Synergistic sorbent separation for one-step ethylene purification from a four-component mixture. Science 366, 241–246 (2019).
Li, L. et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 362, 443–446 (2018).
Howarth, R. W. & Jacobson, M. Z. How green is blue hydrogen? Energy Sci. Eng. 9, 1676–1687 (2021).
Shi, R. et al. Room-temperature electrochemical acetylene reduction to ethylene with high conversion and selectivity. Nat. Catal. 4, 565–574 (2021).
Bu, J. et al. Selective electrocatalytic semihydrogenation of acetylene impurities for the production of polymer-grade ethylene. Nat. Catal. 4, 557–564 (2021).
Schultz, D. M. & Yoon, T. P. Solar synthesis: prospects in visible light photocatalysis. Science 343, 1239176 (2014).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Ciamician, G. The photochemistry of the future. Science 36, 385–394 (1912).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Tavasoli, A. V., Preston, M. & Ozin, G. Photocatalytic dry reforming: what is it good for? Energy Environ. Sci. 14, 3098–3109 (2021).
Zhou, S. et al. Pd single-atom catalysts on nitrogen-doped graphene for the highly selective photothermal hydrogenation of acetylene to ethylene. Adv. Mater. 31, 19005091 (2019).
Swearer, D. F. et al. Heterometallic antenna-reactor complexes for photocatalysis. Proc. Natl Acad. Sci. USA 113, 8916–8920 (2016).
Tokel-Takvoryan, N. E., Hemingway, R. E. & Bard, A. J. Electrogenerated chemiluminescence. XIII. Electrochemical and electrogenerated chemiluminescence studies of ruthenium chelates. J. Am. Chem. Soc. 95, 6582–6589 (1973).
Ghosh, I. et al. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 365, 360–366 (2019).
Savateev, A., Ghosh, I., König, B. & Antonietti, M. Photoredox catalytic organic transformations using heterogeneous carbon nitrides. Angew. Chem. Int. Ed. 57, 15936–15947 (2018).
Call, A. et al. Highly efficient and selective photocatalytic CO2 reduction to CO in water by a cobalt porphyrin molecular catalyst. ACS Catal. 9, 4867–4874 (2019).
Arcudi, F., Dordević, L., Nagasing, B., Stupp, S. I. & Weiss, E. A. Quantum dot-sensitized photoreduction of CO2 in water with turnover number >80,000. J. Am. Chem. Soc. 143, 18131–18138 (2021).
Fleischer, E. B. & Krishnamurthy, M. Reduction of acetylene and nitrogen by a cobalt-porphyrin system. J. Am. Chem. Soc. 94, 1382–1384 (1972).
Ichikawa, M., Sonoda, R. & Meshitsuka, S. Specific catalysis by Co(II)phthalocyanine-tetrasulfonate in the selective reduction of acetylene with sodium boronhydride. Chem. Lett. 2, 709–712 (1973).
Fleischer, E. B. & Krishnamurthy, M. Relationships between porphyrin structure and reactivity. Ann. N. Y. Acad. Sci. 206, 32–46 (1973).
Kojima, M. & Matsunaga, S. The merger of photoredox and cobalt catalysis. Trends Chem. 2, 410–426 (2020).
Bullock, R. M. et al. Using nature’s blueprint to expand catalysis with Earth-abundant metals. Science 369, eabc3183 (2020).
Kohler, L. & Mulfort, K. L. Photoinduced electron transfer kinetics of linked Ru-Co photocatalyst dyads. J. Photochem. Photobiol. A Chem. 373, 59–65 (2019).
Banks, R. G. S., Henderson, R. J. & Pratt, J. M. Reactions of nitrous oxide with some transition-metal complexes. Chem. Commun. 1967, 387–388 (1967).
Symoens, S. H. et al. State-of-the-art of coke formation during steam cracking: anti-coking surface technologies. Ind. Eng. Chem. Res. 57, 16117–16136 (2018).
Pyper, J. W. & Long, F. A. Equilibrium in the deuterium exchange of acetylene and water. J. Chem. Phys. 41, 1890–1896 (1964).
Pyper, J. W. & Liu, D. K. K. Hydrogen-deuterium exchange in acetylene and between acetylene and water. J. Chem. Phys. 67, 845–846 (1977).
Beyene, B. B. & Hung, C. H. Photocatalytic hydrogen evolution from neutral aqueous solution by a water-soluble cobalt(II) porphyrin. Sustain. Energy Fuels 2, 2036–2043 (2018).
Kamei, Y. et al. Silane- and peroxide-free hydrogen atom transfer hydrogenation using ascorbic acid and cobalt-photoredox dual catalysis. Nat. Commun. 12, 966 (2021).
Michiyuki, T. & Komeyama, K. Recent advances in four‐coordinated planar cobalt catalysis in organic synthesis. Asian J. Org. Chem. 9, 343–358 (2020).
Ashley, K. R. & Leipoldt, J. G. Kinetic and equilibrium study of the reaction of (meso-tetrakis(p-sulfonatophenyl)porphyrinato)diaquocobaltate(III) with pyridine in aqueous solution. Inorg. Chem. 20, 2326–2333 (1981).
Dodd, D. β-styrylcobaloximes: mechanism of formation from β-styryl halides and mechanism of cleavage by electrophiles. J. Chem. Soc. Perkin Trans. 2 1976, 1261–1267 (1976).
Gupta, B. D. & Roy, S. Organocobaloximes: cobalt–carbon bond stability and synthesis. Inorg. Chim. Acta 146, 209–221 (1988).
Gridnev, A. A., Ittel, S. D., Fryd, M. & Wayland, B. B. Formation of organocobalt porphyrin complexes by reactions of cobalt(II) porphyrins with azoisobutyronitrile and organic substrates. J. Chem. Soc. J. Chem. Soc. 1993, 1010–1011 (1993).
De Brain, B., Dzik, W. I., Li, S. & Wayland, B. B. Hydrogen-atom transfer in reactions of organic radicals with [CoII(por)] (por = porphyrinato) and in subsequent addition of [Co(H)(por)] to olefins. Eur. J. Chem. A 15, 4312–4320 (2009).
Li, G., Han, A., Pulling, M. E., Estes, D. P. & Norton, J. R. Evidence for formation of a Co–H bond from (H2O)2Co(dmgBF2)2 under H2: application to radical cyclizations. J. Am. Chem. Soc. 134, 14662–14665 (2012).
Delley, M. F. et al. Hydrogen on cobalt phosphide. J. Am. Chem. Soc. 141, 15390–15402 (2019).
Gnaim, S. et al. Cobalt-electrocatalytic HAT for functionalization of unsaturated C–C bonds. Nature 605, 687–695 (2022).
Arnett, R. L. & Crawford, B. L. The vibrational frequencies of ethylene. J. Chem. Phys. 18, 118–126 (1950).
Miller, S. Notes—a comparison of the electrophilic reactivity of styrene and phenylacetylene. J. Org. Chem. 21, 247–248 (1956).
Steinberger, B., Michman, M., Schwarz, H. & Höhne, G. Selective hydrogenation of the CC-triple bond in PhC≡CPh by tris(triphenylphosphine)cobalt activated NaBH4; deuterium tracing experiments. J. Organomet. Chem. 244, 283–288 (1983).
Abdel-Rahman, M. K. & Trenary, M. Propyne hydrogenation over a Pd/Cu(111) single-atom alloy studied using ambient pressure infrared spectroscopy. ACS Catal. 10, 9716–9724 (2020).
Ardo, S., Achey, D., Morris, A. J., Abrahamsson, M. & Meyer, G. J. Non-Nernstian two-electron transfer photocatalysis at metalloporphyrin–TiO2 interfaces. J. Am. Chem. Soc. 133, 16572–16580 (2011).
Acknowledgements
This work was supported by the Center for Bio-Inspired Energy Science (CBES), an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences, under award no. DE-SC0000989. This work made use of the IMSERC facility at Northwestern University, which has received support from the NIH (1S10OD012016-01/1S10RR019071-01A1), the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205), the State of Illinois and the International Institute for Nanotechnology (IIN), and the REACT Core facility at Northwestern University, funded by the US Department of Energy, Catalysis Science programme (DE-SC0001329) for the purchase of the GC-MS instrument. We thank S. Alayoglu and R. López-Arteaga for help with the gas-phase IR and emission lifetime measurements, respectively.
Dedication: We dedicate this work to Sir Fraser Stoddart on the occasion of his 80th birthday.
Author information
Authors and Affiliations
Contributions
F.A., L.Ð. and E.A.W. conceived the project, contributed to the experimental design and wrote the manuscript. E.A.W. directed the research. F.A. and L.Ð. designed and performed the experiments and analysed the results. F.A., L.Ð. and N.S. carried out the GC experiments. L.Ð. and S.I.S. designed and prepared the materials. E.A.W. and S.I.S. secured the funding. All authors contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
F.A., L.Ð., E.A.W. and S.I.S. are co-inventors of a patent application (no. PCT/US2022/026732) filed by Northwestern University on the photocatalytic reduction of acetylene to ethylene.
Peer review
Peer review information
Nature Chemistry thanks Sven Rau, Tierui Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental Section, Supplementary Figs. 1–24, Tables 1–6 and references.
Source data
Source Data Fig. 2
Underlying measured data
Source Data Fig. 3
Underlying measured data
Source Data Fig. 4
Underlying measured data
Rights and permissions
About this article
Cite this article
Arcudi, F., Ðorđević, L., Schweitzer, N. et al. Selective visible-light photocatalysis of acetylene to ethylene using a cobalt molecular catalyst and water as a proton source. Nat. Chem. 14, 1007–1012 (2022). https://doi.org/10.1038/s41557-022-00966-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00966-5
This article is cited by
-
A triple tandem reaction for the upcycling of products from poorly selective CO2 photoreduction systems
Nature Synthesis (2024)
-
Adaptive alkyne trap purifies crude ethylene
Nature Chemical Engineering (2024)
-
Solar reforming as an emerging technology for circular chemical industries
Nature Reviews Chemistry (2024)
-
Non-noble metal-based catalysts for acetylene semihydrogenation: from thermocatalysis to sustainable catalysis
Science China Chemistry (2023)