Abstract
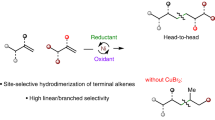
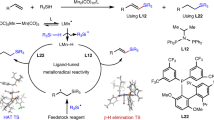
The controlled isomerization and functionalization of alkenes is a cornerstone achievement in organometallic catalysis that is now widely used throughout industry. In particular, the addition of CO and H2 to an alkene, also known as the oxo-process, is used in the production of linear aldehydes from crude alkene feedstocks. In these catalytic reactions, isomerization is governed by thermodynamics, giving rise to functionalization at the most stable alkylmetal species. Despite the ubiquitous industrial applications of tandem alkene isomerization/functionalization reactions, selective functionalization at internal positions has remained largely unexplored. Here we report that the simple W(0) precatalyst W(CO)6 catalyses the isomerization of alkenes to unactivated internal positions and subsequent hydrocarbonylation with CO. The six- to seven-coordinate geometry changes that are characteristic of the W(0)/W(II) redox cycle and the conformationally flexible directing group are key factors in allowing isomerization to take place over multiple positions and stop at a defined unactivated internal site that is primed for in situ functionalization.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this article (and its Supplementary Information). The structures of W-2, W-3, W-3′, W-4 and 2u in the solid state were determined by single-crystal X-ray diffraction and the crystallographic data have been deposited with the Cambridge Crystallographic Data Centre: CCDC 2020047 (W-2), 2050458 (W-3), 2045639 (W-3′), 2110274 (W-4), 2008992 (2u). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Trnka, T. M. & Grubbs, R. H. The development of L2X2Ru=CHR olefin metathesis catalysts: an organometallic success story. Acc. Chem. Res. 34, 18–29 (2001).
Schrock, R. R. Multiple metal–carbon bonds for catalytic metathesis reactions (Nobel lecture). Angew. Chem. Int. Ed. 45, 3748–3759 (2006).
Masuda, T. & Higashimura, T. Synthesis of high polymers from substituted acetylenes: exploitation of molybdenum- and tungsten-based catalysts. Acc. Chem. Res. 17, 51–56 (1984).
Liebov, B. K. & Harman, W. D. Group 6 dihapto-coordinate dearomatization agents for organic synthesis. Chem. Rev. 117, 13721–13755 (2017).
Smith, J. A. et al. Preparation of cyclohexene isotopologues and stereoisotopomers from benzene. Nature 581, 288–293 (2020).
Adrjan, B. & Szymanska-Buzar, T. Photochemical reactions of [W(CO)4(η4-nbd)] with hydrosilanes: generation of new hydrido complexes of tungsten and their reactivity. J. Organomet. Chem. 693, 2163–2170 (2008).
Handzlik, J., Kochel, A. & Szymańska-Buzar, T. H–Ge bond activation by tungsten carbonyls: an experimental and theoretical study. Polyhedron 31, 622–631 (2012).
Chakraborty, S. & Berke, H. Homogeneous hydrogenation of nitriles catalyzed by molybdenum and tungsten amides. ACS Catal. 4, 2191–2194 (2014).
Wrighton, M., Hammond, G. S. & Gray, H. B. Group VI metal carbonyl photoassisted isomerization of olefins. J. Organomet. Chem. 70, 283–301 (1974).
Moberg, C. in Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis (ed. Kazmaier, U.) 209–234 (Springer, 2011).
Hoffmann, R., Beier, B. F., Muetterties, E. L. & Rossi, A. R. Seven-coordination. A molecular orbital exploration of structure, stereochemistry, and reaction dynamics. Inorg. Chem. 16, 511–522 (1977).
Hoffmann, R., Wilker, C. N., Lippard, S. J., Templeton, J. L. & Bower, D. C. Theoretical prescription for reductive coupling of CO or CNR ligands. J. Am. Chem. Soc. 105, 146–147 (1983).
Lam, C. T., Corfield, P. W. R. & Lippard, S. J. Reductive coupling of adjacent ligands in a seven-coordinate molybdenum(II) isocyanide complex. J. Am. Chem. Soc. 99, 617–618 (1977).
Giandomenico, C. M., Lam, C. T. & Lippard, S. J. Reductive coupling of coordinated alkyl isocyanides in seven-coordinate molybdenum(II) and tungsten(II) complexes. Removal of the coupled ligand as an oxamide. J. Am. Chem. Soc. 104, 1263–1271 (1982).
Kazlauskas, R. J. & Wrighton, M. S. Photogeneration of intermediates involved in catalytic cycles. β-Hydride elimination from the 16-electron alkyl species generated by irradiation of tricarbonyl(η5-cyclopentadienyl)(n-pentyl)tungsten(II). J. Am. Chem. Soc. 102, 1727–1730 (1980).
Kazlauskas, R. J. & Wrighton, M. S. Photochemistry of metal carbonyl alkyls. Study of thermal β-hydrogen transfer in photogenerated, 16-valence-electron alkyldicarbonylcyclopentadienylmolybdenum and -tungsten complexes. J. Am. Chem. Soc. 104, 6005–6015 (1982).
Vilches-Herrera, M., Domke, L. & Börner, A. Isomerization–hydroformylation tandem reactions. ACS Catal. 4, 1706–1724 (2014).
Franke, R., Selent, D. & Börner, A. Applied hydroformylation. Chem. Rev. 112, 5675–5732 (2012).
Whiteker, G. T., Cobley, C. J. in Organometallics as Catalysts in the Fine Chemical Industry (eds Beller, M. & Blaser, H. U.) 35–46 (Springer, 2012).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Sommer, H., Juliá-Hernández, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Kochi, T., Hamasaki, T., Aoyama, Y., Kawasaki, J. & Kakiuchi, F. Chain-walking strategy for organic synthesis: catalytic cycloisomerization of 1,n-dienes. J. Am. Chem. Soc. 134, 16544–16547 (2012).
Sommer, H., Weissbrod, T. & Marek, I. A tandem iridium-catalyzed “chain-walking”/Cope rearrangement sequence. ACS Catal. 9, 2400–2406 (2019).
Jun, C.-H., Lee, H. & Lim, S.-G. The C-C bond activation and skeletal rearrangement of cycloalkanone imine by Rh(I) catalysts. J. Am. Chem. Soc. 123, 751–752 (2001).
O’Duill, M. L. et al. Tridentate directing groups stabilize 6-membered palladacycles in catalytic alkene hydrofunctionalization. J. Am. Chem. Soc. 139, 15576–15579 (2017).
McCusker, J. E., Logan, J. & McElwee-White, L. Oxidative carbonylation of primary amines to ureas using tungsten carbonyl catalysts. Organometallics 17, 4037–4041 (1998).
Breit, B. in Directed Metallation (ed. Chatani, N.) 145–168 (Springer, 2007).
Chen, X., Rao, W., Yang, T. & Koh, M. J. Alkyl halides as both hydride and alkyl sources in catalytic regioselective reductive olefin hydroalkylation. Nat. Commun. 11, 5857 (2020).
Lv, H. et al. Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation. Nat. Commun. 10, 5025 (2019).
Inoue, S., Shiota, H., Fukumoto, Y. & Chatani, N. Ruthenium-catalyzed carbonylation at ortho C–H bonds in aromatic amides leading to phthalimides: C–H bond activation utilizing a bidentate system. J. Am. Chem. Soc. 131, 6898–6899 (2009).
Wrighton, M. Photochemistry of metal carbonyls. Chem. Rev. 74, 401–430 (1974).
Ditri, T. B., Moore, C. E., Rheingold, A. L. & Figueroa, J. S. Oxidative decarbonylation of m-terphenyl isocyanide complexes of molybdenum and tungsten: precursors to low-coordinate isocyanide complexes. Inorg. Chem. 50, 10448–10459 (2011).
Corey, E. J., Cheng, X. M. The Logic of Chemical Synthesis (Wiley, 1989).
Darensbourg, D. J. et al. A kinetic investigation of carbon dioxide insertion processes involving anionic tungsten-alkyl and -aryl derivatives: effects of carbon dioxide pressure, counterions, and ancillary ligands. Comparisons with migratory carbon monoxide insertion processes. J. Am. Chem. Soc. 107, 7463–7473 (1985).
Buffin, B. P., Poss, M. J., Arif, A. M. & Richmond, T. G. Synthesis and reactivity of a tungsten(0) anion stabilized by chelating tertiary amines. The oxidative addition and reductive elimination of a carbon–tin bond at tungsten. Inorg. Chem. 32, 3805–3806 (1993).
Biswas, S. Mechanistic understanding of transition-metal-catalyzed olefin isomerization: metal-hydride insertion–elimination vs. π-allyl pathways. Comments Inorg. Chem. 35, 300–330 (2015).
Szymańska-Buzar, T., Jaroszewski, M., Wilgocki, M. & Ziółkowski, J. J. Reactivity of bis(alkene) tetracarbonyl complexes of tungsten: evidence for alkene to π-allyl hydride rearrangement. J. Mol. Cat. A 112, 203–210 (1996).
Sheng, Y., Musaev, D. G., Reddy, K. S., McDonald, F. E. & Morokuma, K. Computational studies of tungsten-catalyzed endo-selective cycloisomerization of 4-pentyn-1-ol. J. Am. Chem. Soc. 124, 4149–4157 (2002).
Kochi, T., Kanno, S. & Kakiuchi, F. Nondissociative chain walking as a strategy in catalytic organic synthesis. Tetrahedron Lett. 60, 150938 (2019).
Jankins, T., Martin-Montero, R., Coopper, P., Martin, R. & Engle, K. M. Low-valent tungsten catalysis enables site-selective isomerization–hydroboration of unactivated alkenes. J. Am. Chem. Soc. 143, 14981–14986 (2021).
Acknowledgements
B. Sanchez and E. Sturgell (Scripps Research Automated Synthesis Facility) are acknowledged for HPLC and chiral SFC analysis. H. Nguyen, K. McClymont, T. Huffman and C. Bi are acknowledged for donation of various starting materials. We also thank J. Vantourout, C. Landis, J. Figueroa and H. Renata for helpful discussions. Financial support for this work was provided by the National Institutes of Health R35GM125052 (K.M.E.), R35GM128779 (P.L) and 1S10OD025208 (J.S.C.). We acknowledge USTC for sponsoring Z.-Y.Q. with a summer exchange scholarship. DFT calculations were performed at the Center for Research Computing at the University of Pittsburgh, the Frontera supercomputer at the Texas Advanced Computing Center, and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the NSF.
Author information
Authors and Affiliations
Contributions
T.C.J. carried out all the experiments and data analysis. Z.-Y.Q. carried out synthesis of various alkene starting materials. J.S.C. helped design and perform pressurized experiments. M.G. carried out collection and analysis of X-ray data. W.C.B, Y.Z. and P.L. carried out computation work. T.C.J., P.L. and K.M.E. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Graham Dobereiner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Data regarding reaction optimization, synthetic and experimental procedures, full characterization data including NMR spectra X-ray crystallographic data, 26 supplementary figures, and 38 supplementary tables.
Supplementary Data 1
xyz coordinates for DFT calculations.
Supplementary Data 2
Original NMR data in MNova format.
Supplementary Data 3
Crystallographic data for compound W-2; CCDC reference 2020047.
Supplementary Data 4
Crystallographic data for compound W-3; CCDC reference 2050458.
Supplementary Data 5
Crystallographic data for compound W-3’; CCDC reference 2045639.
Supplementary Data 6
Crystallographic data for compound W-4; CCDC reference 2110274.
Supplementary Data 7
Crystallographic data for compound 2u; CCDC 2008992.
Rights and permissions
About this article
Cite this article
Jankins, T.C., Bell, W.C., Zhang, Y. et al. Low-valent tungsten redox catalysis enables controlled isomerization and carbonylative functionalization of alkenes. Nat. Chem. 14, 632–639 (2022). https://doi.org/10.1038/s41557-022-00951-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00951-y
This article is cited by
-
Divergent regioselective Heck-type reaction of unactivated alkenes and N-fluoro-sulfonamides
Nature Communications (2022)
-
Tungsten’s tandem transformation
Nature Chemistry (2022)