Abstract
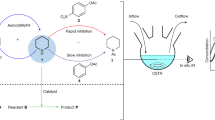
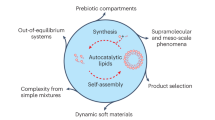
A key goal of chemistry is to develop synthetic systems that mimic biology, such as self-assembling, self-replicating models of minimal life forms. Oscillations are often observed in complex biological networks, but oscillating, self-replicating species are unknown, and how to control autonomous supramolecular-level oscillating systems is also not yet established. Here we show how a population of self-assembling self-replicators can autonomously oscillate, so that simple micellar species repeatedly appear and disappear in time. The interplay of molecular and supramolecular events is key to observing oscillations: the repeated formation and disappearance of compartments is connected to a reaction network where molecular-level species are formed and broken down. The dynamic behaviour of our system across different length scales offers the opportunities for mass transport, as we demonstrate via reversible dye uptake. We believe these findings will inspire new biomimetic systems and may unlock nanotechnology systems such as (supra)molecular pumps, where compartment formation is controlled in time and space.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The source data underlying Figs. 1b, 2b, 3a–e and 4 are provided as a source data file. Source data are provided with this paper.
References
Goldbeter, A. Biochemical Oscillations and Cellular Rhythms (Cambridge Univ. Press, 1996).
Cao, Y. X. L., Lopatkin, A. & You, L. C. Elements of biological oscillations in time and space. Nat. Struct. Mol. Biol. 23, 1030–1034 (2016).
Ruiz-Mirazo, K., Briones, C. & de la Escosura, A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 114, 285–366 (2014).
Lotka, A. J. Undamped oscillations derived from the law of mass action. J. Am. Chem. Soc. 42, 1595–1599 (1920).
Volterra, V. Fluctuations in the abundance of a species considered mathematically. Nature 118, 558–560 (1926).
Epstein, I. R. & Showalter, K. Nonlinear chemical dynamics: oscillations, patterns, and chaos. J. Phys. Chem. 100, 13132–13147 (1996).
van Rossum, S. A. P., Tena-Solsona, M., van Esch, J. H., Eelkema, R. & Boekhoven, J. Dissipative out-of-equilibrium assembly of man-made supramolecular materials. Chem. Soc. Rev. 46, 5519–5535 (2017).
Ashkenasy, G., Hermans, T. M., Otto, S. & Taylor, A. F. Systems chemistry. Chem. Soc. Rev. 46, 2543–2554 (2017).
Kim, Y. S., Tamate, R., Akimoto, A. M. & Yoshida, R. Recent developments in self-oscillating polymeric systems as smart materials: from polymers to bulk hydrogels. Mater. Horiz. 4, 38–54 (2017).
Chen, I. C. et al. Shape- and size-dependent patterns in self-oscillating polymer gels. Soft Matter 7, 3141–3146 (2011).
Yoshizawa, T. et al. Fabrication of self-oscillating micelles with a built-in oxidizing agent. Angew. Chem. Int. Ed. 59, 3871–3875 (2020).
Wang, G. T. et al. The fabrication of a supra-amphiphile for dissipative self-assembly. Chem. Sci. 7, 1151–1155 (2016).
Lagzi, I., Kowalczyk, B., Wang, D. W. & Grzybowski, B. A. Nanoparticle oscillations and fronts. Angew. Chem. Int. Ed. 49, 8616–8619 (2010).
Semenov, S. N. et al. Rational design of functional and tunable oscillating enzymatic networks. Nat. Chem. 7, 160–165 (2015).
Semenov, S. N. et al. Autocatalytic, bistable, oscillatory networks of biologically relevant organic reactions. Nature 537, 656–660 (2016).
Cafferty, B. J. et al. Robustness, entrainment, and hybridization in dissipative molecular networks, and the origin of life. J. Am. Chem. Soc. 141, 8289–8295 (2019).
Wagner, N. & Ashkenasy, G. Rhythm before life. Nat. Chem. 11, 681–683 (2019).
Leira-Iglesias, J., Tassoni, A., Adachi, T., Stich, M. & Hermans, T. M. Oscillations, travelling fronts and patterns in a supramolecular system. Nat. Nanotechnol. 13, 1021–1027 (2018).
Green, L. N. et al. Autonomous dynamic control of DNA nanostructure self-assembly. Nat. Chem. 11, 510–520 (2019).
Srinivas, N., Parkin, J., Seelig, G., Winfree, E. & Soloveiehile, D. Enzyme-free nucleic acid dynamical systems. Science 358, eaal2052 (2017).
Morrow, S. M., Colomer, I. & Fletcher, S. P. A chemically fuelled self-replicator. Nat. Commun. 10, 1011 (2019).
Lebedeva, M. A., Palmieri, E., Kukura, P. & Fletcher, S. P. Emergence and rearrangement of dynamic supramolecular aggregates visualized by interferometric scattering microscopy. ACS Nano 14, 11160–11168 (2020).
Bachmann, P. A., Luisi, P. L. & Lang, J. Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 357, 57–59 (1992).
Bissette, A. J. & Fletcher, S. P. Mechanisms of Autocatalysis. Angew. Chem. Int. Ed. 52, 12800–12826 (2013).
Engwerda, A. H. J. et al. Coupled metabolic cycles allow out-of-equilibrium autopoietic vesicle replication. Angew. Chem. Int. Ed. 59, 20361–20366 (2020).
Kukura, P. et al. High-speed nanoscopic tracking of the position and orientation of a single virus. Nat. Methods 6, 923–927 (2009).
Young, G. et al. Quantitative mass imaging of single biological macromolecules. Science 360, 423–427 (2018).
Szostak, J. W., Bartel, D. P. & Luisi, P. L. Synthesizing life. Nature 409, 387–390 (2001).
Dzieciol, A. J. & Mann, S. Designs for life: protocell models in the laboratory. Chem. Soc. Rev. 41, 79–85 (2012).
Plys, A. J. & Kingston, R. E. Dynamic condensates activate transcription Transcriptional components exhibit transient phase separation to drive gene activation. Science 361, 329–330 (2018).
Altenburg, W. J. et al. Programmed spatial organization of biomacromolecules into discrete, coacervate-based protocells. Nat. Commun. 11, 6282 (2020).
Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 (2018).
Boeynaems, S. et al. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 28, 420–435 (2018).
Taylor, R. C., Cullen, S. P. & Martin, S. J. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 9, 231–241 (2008).
Grzybowski, B. A. & Huck, W. T. S. The nanotechnology of life-inspired systems. Nat. Nanotechnol. 11, 584–591 (2016).
He, X. M. et al. Synthetic homeostatic materials with chemo-mechano-chemical self-regulation. Nature 487, 214–218 (2012).
Lerch, M. M., Grinthal, A. & Aizenberg, J. Viewpoint: homeostasis as inspiration-toward interactive materials. Adv. Mater. 32, 1905554 (2020).
Hwang, I. et al. Audible sound-controlled spatiotemporal patterns in out-of-equilibrium systems. Nat. Chem. 12, 808–813 (2020).
Mandelkow, E., Mandelkow, E. M., Hotani, H., Hess, B. & Muller, S. C. Spatial patterns from oscillating microtubules. Science 246, 1291–1293 (1989).
Acknowledgements
The authors wish to thank E. Foley for help with iSCAT measurements and L. Carrique for performing cryo-transmission electron microscopy measurements. The authors thank the ERC (Consolidator Grant, Autocat, 681491) for funding. M.G.H. thanks the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) for a studentship, generously supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB and Vertex. This work used the OPIC electron microscopy facility which is supported by a Wellcome JIF award (060208/Z/00/Z) and a Wellcome equipment grant (093305/Z/10/Z).
Author information
Authors and Affiliations
Contributions
M.G.H. and A.H.J.E. performed the experiments. R.J.H.S. performed preliminary experiments. M.G.H., A.H.J.E. and S.P.F. contributed to designing and analysing the experiments and writing and editing the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods and synthesis, discussion and additional data.
Supplementary Video 1
Video of the visual oscillations seen when incorporating a hydrophobic dye (250× speed). Reaction is sampled for UPLC analysis.
Supplementary Video 2
Video of the visual oscillations seen when incorporating a hydrophobic dye (duplicate reaction, 250× speed). Reaction is not sampled or disturbed.
Source data
Source Data Fig. 1
Raw numerical data for graph(s) in Figure 1
Source Data Fig. 2
Raw numerical data for graph(s) in Figure 2
Source Data Fig. 3
Raw numerical data for graph(s) in Figure 3
Source Data Fig. 4
Raw numerical data for graph(s) in Figure 4
Rights and permissions
About this article
Cite this article
Howlett, M.G., Engwerda, A.H.J., Scanes, R.J.H. et al. An autonomously oscillating supramolecular self-replicator. Nat. Chem. 14, 805–810 (2022). https://doi.org/10.1038/s41557-022-00949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00949-6
This article is cited by
-
Selenium catalysis enables negative feedback organic oscillators
Nature Communications (2024)
-
Autocatalytic flow chemistry
Scientific Reports (2023)
-
From autocatalysis to survival of the fittest in self-reproducing lipid systems
Nature Reviews Chemistry (2023)
-
Mechanosensitive non-equilibrium supramolecular polymerization in closed chemical systems
Nature Communications (2023)
-
A catalytically active oscillator made from small organic molecules
Nature (2023)